Dual-targeted and pH-sensitive Doxorubicin Prodrug-Microbubble Complex with Ultrasound for Tumor Treatment
- PMID: 28255342
- PMCID: PMC5327360
- DOI: 10.7150/thno.16677
Dual-targeted and pH-sensitive Doxorubicin Prodrug-Microbubble Complex with Ultrasound for Tumor Treatment
Abstract
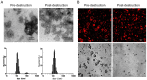
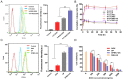
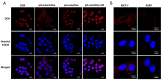
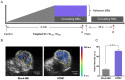
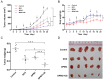
In this study, we investigated the potential of a dual-targeted pH-sensitive doxorubicin prodrug-microbubble complex (DPMC) in ultrasound (US)-assisted antitumor therapy. The doxorubicin prodrug (DP) consists of a succinylated-heparin carrier conjugated with doxorubicin (DOX) via hydrazone linkage and decorated with dual targeting ligands, folate and cRGD peptide. Combination of microbubble (MB) and DP, generated via avidin-biotin binding, promoted intracellular accumulation and improved therapeutic efficiency assisted by US cavitation and sonoporation. Aggregates of prepared DP were observed with an inhomogeneous size distribution (average diameters: 149.6±29.8 nm and 1036.2±38.8 nm, PDI: 1.0) while DPMC exhibited a uniform distribution (average diameter: 5.804±2.1 μm), facilitating its usage for drug delivery. Notably, upon US exposure, DPMC was disrupted and aggregated DP dispersed into homogeneous small-sized nanoparticles (average diameter: 128.6±42.3 nm, PDI: 0.21). DPMC could target to angiogenic endothelial cells in tumor region via αvβ3-mediated recognition and subsequently facilitate its specific binding to tumor cells mediated via recognition of folate receptor (FR) after US exposure. In vitro experiments showed higher tumor specificity and killing ability of DPMC with US than free DOX and DP for breast cancer MCF-7 cells. Furthermore, significant accumulation and specificity for tumor tissues of DPMC with US were detected using in vivo fluorescence and ultrasound molecular imaging, indicating its potential to integrate tumor imaging and therapy. In particular, through inducing apoptosis, inhibiting cell proliferation and antagonizing angiogenesis, DPMC with US produced higher tumor inhibition rates than DOX or DPMC without US in MCF-7 xenograft tumor-bearing mice while inducing no obvious body weight loss. Our strategy provides an effective platform for the delivery of large-sized or aggregated particles to tumor sites, thereby extending their therapeutic applications in vivo.
Keywords: Doxorubicin prodrug; Dual-targeted; Microbubble complex; Ultrasound.; pH-sensitive.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X. et al. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–35. doi: 10.1016/j.biomaterials.2014.10.007. - PubMed
-
- Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Gold nanoparticles with a monolayer of doxorubicin-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. Biomaterials. 2009;30:6065–75. doi:10.1016/j.biomaterials.2009.07.048. - PubMed
-
- She W, Luo K, Zhang C, Wang G, Geng Y, Li L. et al. The potential of self-assembled, pH-responsive nanoparticles of mPEGylated peptide dendron-doxorubicin conjugates for cancer therapy. Biomaterials. 2013;34:1613–23. doi: 10.1016/j.biomaterials.2012.11.007. - PubMed
-
- Chakravarty R, Chakraborty S, Dash A. Molecular Imaging of Breast Cancer: Role of RGD Peptides. Mini Rev Med Chem. 2015;15:1073–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

