Expression Analysis of Hairpin RNA Carrying Sugarcane mosaic virus (SCMV) Derived Sequences and Transgenic Resistance Development in a Model Rice Plant
- PMID: 28255554
- PMCID: PMC5309402
- DOI: 10.1155/2017/1646140
Expression Analysis of Hairpin RNA Carrying Sugarcane mosaic virus (SCMV) Derived Sequences and Transgenic Resistance Development in a Model Rice Plant
Abstract
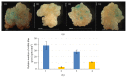
Developing transgenic resistance in monocotyledonous crops against pathogens remains a challenging area of research. Sugarcane mosaic virus (SCMV) is a serious pathogen of many monocotyledonous crops including sugarcane. The objective of present study was to analyze transgenic expression of hairpin RNA (hpRNA), targeting simultaneously CP (Coat Protein) and Hc-Pro (helper component-proteinase) genes of SCMV, in a model rice plant. Conserved nucleotide sequences, exclusive for DAG (Aspartic acid-Alanine-Glycine) and KITC (Lycine-Isoleucine-Threonine-Cysteine) motifs, derived from SCMV CP and Hc-Pro genes, respectively, were fused together and assembled into the hpRNA cassette under maize ubiquitin promoter to form Ubi-hpCP:Hc-Pro construct. The same CP:Hc-Pro sequence was fused with the β-glucuronidase gene (GUS) at the 3' end under CaMV 35S promoter to develop 35S-GUS:CP:Hc-Pro served as a target reporter gene construct. When delivered into rice callus tissues by particle bombardment, the Ubi-hpCP:Hc-Pro construct induced strong silencing of 35S-GUS:CP:Hc-Pro. Transgenic rice plants, containing Ubi-hpCP:Hc-Pro construct, expressed high level of 21-24 nt small interfering RNAs, which induced specific suppression against GUS:CP:Hc-Pro delivered by particle bombardment and conferred strong resistance to mechanically inoculated SCMV. It is concluded that fusion hpRNA approach is an affordable method for developing resistance against SCMV in model rice plant and it could confer SCMV resistance when transformed into sugarcane.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
-
- Anwar S. Impact of sugarcane disease on cane and sugar yield. Proceedings of the 3rd Agricultural Biotechnology Workshop; 2005; Faisalabad, Pakistan. Agricultural Biotechnology Research Institute;
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

