GSK-3β inhibitor TDZD-8 reduces neonatal hypoxic-ischemic brain injury in mice
- PMID: 28256059
- PMCID: PMC6492674
- DOI: 10.1111/cns.12683
GSK-3β inhibitor TDZD-8 reduces neonatal hypoxic-ischemic brain injury in mice
Abstract
Aims: Glycogen synthase kinase 3β (GSK-3β) is activated following hypoxic-ischemic (HI) brain injury. TDZD-8 is a specific GSK-3β inhibitor. Currently, the impact of inhibiting GSK-3β in neonatal HI injury is unknown. We aimed to investigate the effect of TDZD-8 following neonatal HI brain injury.
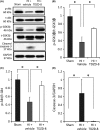
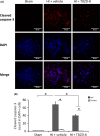
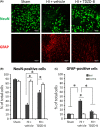
Methods: Unilateral common carotid artery ligation followed by hypoxia was used to induce HI injury in postnatal day 7 mouse pups pretreated with TDZD-8 or vehicle. The infarct volume, whole-brain imaging, Nissl staining, and behavioral tests were used to evaluate the protective effect of TDZD-8 on the neonatal brain and assess functional recovery after injury. Western blot was used to evaluate protein levels of phosphorylated protein kinase B (Akt), GSK-3β, and cleaved caspase-3. Protein levels of cleaved caspase-3, neuronal marker, and glial fibrillary acidic protein were detected through immunohistochemistry.
Results: Pretreatment with TDZD-8 significantly reduced brain damage and improved neurobehavioral outcomes following HI injury. TDZD-8 reversed the reduction of phosphorylated Akt and GSK-3β, and the activation of caspase-3 induced by hypoxia-ischemia. In addition, TDZD-8 suppressed apoptotic cell death and reduced reactive astrogliosis.
Conclusion: TDZD-8 has the therapeutic potential for hypoxic-ischemic brain injury in neonates. The neuroprotective effect of TDZD-8 appears to be mediated through its antiapoptotic activity and by reducing astrogliosis.
Keywords: GSK-3β; TDZD-8; neonatal hypoxia-ischemia, neuroprotection.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Yao HB, Shaw PC, Wong CC, et al. Expression of glycogen synthase kinase‐3 isoforms in mouse tissues and their transcription in the brain. J Chem Neuroanat. 2002;23:291‐297. - PubMed
-
- Mukai F, Ishiguro K, Sano Y, et al. Alternative splicing isoform of tau protein kinase I/glycogen synthase kinase 3 beta. J Neurochem. 2002;81:1073‐1083. - PubMed
-
- Shi SH, Cheng T, Jan LY, et al. APC and GSK‐3 beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14:2025‐2032. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

