A quantitative structural and morphometric analysis of the Purkinje network and the Purkinje-myocardial junctions in pig hearts
- PMID: 28256093
- PMCID: PMC5382594
- DOI: 10.1111/joa.12594
A quantitative structural and morphometric analysis of the Purkinje network and the Purkinje-myocardial junctions in pig hearts
Abstract
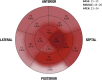
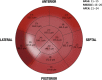
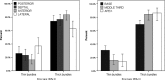
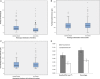
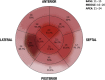
The morpho-functional properties of the distal section of the cardiac Purkinje network (PN) and the Purkinje-myocardial junctions (PMJs) are fundamental to understanding the sequence of electrical activation in the heart. The overall structure of the system has already been described, and several computational models have been developed to gain insight into its involvement in cardiac arrhythmias or its interaction with implantable devices, such as pacemakers. However, anatomical descriptions of the PN in the literature have not enabled enough improvements in the accuracy of anatomical-based electrophysiological simulations of the PN in 3D hearts models. In this work, we study the global distribution and morphological properties of the PN, with special emphasis on the cellular and architectural characterization of its intramural branching structure, mesh-like sub-endocardial network, and the PMJs in adult pig hearts by both histopathological and morphometric evaluation. We have defined three main patterns of PMJ: contact through cell bodies, contact through cell prolongations either thick or piliform, and contact through transitional cells. Moreover, from hundreds of micrographs, we quantified the density of PMJs and provided data for the basal/medial/apical regions, anterior/posterior/septal/lateral regions and myocardial/sub-endocardial distribution. Morphometric variables, such as Purkinje cell density and thickness of the bundles, were also analyzed. After combining the results of these parameters, a different septoanterior distribution in the Purkinje cell density was observed towards the cardiac apex, which is associated with a progressive thinning of the conduction bundles and the posterolateral ascension of intramyocardial terminal scattered fibers. The study of the PMJs revealed a decreasing trend towards the base that may anatomically explain the early apical activation. The anterolateral region contains the greatest number of contacts, followed by the anterior and septal regions. This supports the hypothesis that thin distal Purkinje bundles create a junction-rich network that may be responsible for the quick apical depolarization. The PN then ascends laterally and spreads through the anterior and medial walls up to the base. We have established the first morphometric study of the Purkinje system, and provided quantitative and objective data that facilitate its incorporation into the development of models beyond gross and variable pathological descriptions, and which, after further studies, could be useful in the characterization of pathological processes or therapeutic procedures.
Keywords: Purkinje quantitative analysis; Purkinje-myocardial junction; cardiac conduction system; morphometry; pig heart.
© 2017 Anatomical Society.
Figures





References
-
- Abouezzeddine O, Suleiman M, Buescher T, et al. (2010) Relevance of endocavitary structures in ablation procedures for ventricular tachycardia. J Cardiovasc Electrophysiol 21, 245–254. - PubMed
-
- Ansari A, Yen Ho S, Anderson R (1999) Distribution of the Purkinje fibres in the sheep heart. Anat Rec 254, 92–97. - PubMed
-
- Atkinson A, Inada S, Li J, et al. (2011) Anatomical and molecular mapping of the left and right ventricular His‐Purkinje conduction networks. J Mol Cell Cardiol 51, 689–701. - PubMed
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

