Autophagy down regulates pro-inflammatory mediators in BV2 microglial cells and rescues both LPS and alpha-synuclein induced neuronal cell death
- PMID: 28256519
- PMCID: PMC5335665
- DOI: 10.1038/srep43153
Autophagy down regulates pro-inflammatory mediators in BV2 microglial cells and rescues both LPS and alpha-synuclein induced neuronal cell death
Abstract
Autophagy is a fundamental cellular homeostatic mechanism, whereby cells autodigest parts of their cytoplasm for removal or turnover. Neurodegenerative disorders are associated with autophagy dysregulation, and drugs modulating autophagy have been successful in several animal models. Microglial cells are phagocytes in the central nervous system (CNS) that become activated in pathological conditions and determine the fate of other neural cells. Here, we studied the effects of autophagy on the production of pro-inflammatory molecules in microglial cells and their effects on neuronal cells. We observed that both trehalose and rapamycin activate autophagy in BV2 microglial cells and down-regulate the production of pro-inflammatory cytokines and nitric oxide (NO), in response to LPS and alpha-synuclein. Autophagy also modulated the phosphorylation of p38 and ERK1/2 MAPKs in BV2 cells, which was required for NO production. These actions of autophagy modified the impact of microglial activation on neuronal cells, leading to suppression of neurotoxicity. Our results demonstrate a novel role for autophagy in the regulation of microglial cell activation and pro-inflammatory molecule secretion, which may be important for the control of inflammatory responses in the CNS and neurotoxicity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
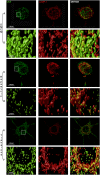
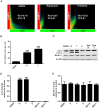

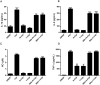
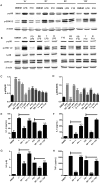
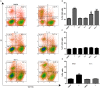
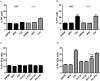
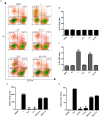
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

