Physico-mechanical and morphological features of zirconia substituted hydroxyapatite nano crystals
- PMID: 28256557
- PMCID: PMC5335334
- DOI: 10.1038/srep43202
Physico-mechanical and morphological features of zirconia substituted hydroxyapatite nano crystals
Abstract
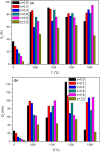
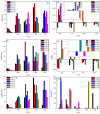
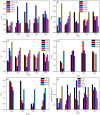
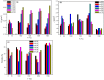
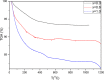
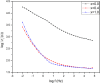
Zirconia doped Hydroxyapatite (HAP) nanocrystals [Ca10(PO4)6-x(ZrO2)x(OH)2]; (0 ≤ x ≤ 1 step 0.2) were synthesized using simple low cost facile method. The crystalline phases were examined by X-ray diffraction (XRD). The crystallinity percentage decreased with increasing zirconia content for the as-synthesized samples. The existence of zirconia as secondary phase on the grain boundaries; as observed from scanning electron micrographs (FESEM); resulted in negative values of microstrain. The crystallite size was computed and the results showed that it increased with increasing annealing temperature. Thermo-gravimetric analysis (TGA) assured the thermal stability of the nano crystals over the temperature from room up to 1200 °C depending on the zirconia content. The corrosion rate was found to decrease around 25 times with increasing zirconia content from x = 0.0 to 1.0. Microhardness displayed both compositional and temperature dependence. For the sample (x = 0.6), annealed at 1200 °C, the former increased up to 1.2 times its original value (x = 0.0).
Conflict of interest statement
The authors declare no competing financial interests.
Figures










References
-
- Zhou H. & Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta biomat. 7, 2769–2781 (2011). - PubMed
-
- Wang M.-C. et al.. Crystalline size, microstructure and biocompatibility of hydroxyapatite nanopowders by hydrolysis of calcium hydrogen phosphate dehydrate (DCPD). Ceram. Int. 41, 2999–3008 (2015).
-
- Kolmas J. et al.. Incorporation of carbonate and magnesium ions into synthetic hydroxyapatite: The effect on physicochemical properties. J. Mol. Struc. 987, 40–50 (2011).
-
- Ramesh S., Tan C. Y., Peralta C. L. & Teng W. D. The effect of manganese oxide on the sinterability of hydroxyapatite. Sci. Tech. Adv. Mat. 8, 257–263 (2007).
LinkOut - more resources
Full Text Sources
Other Literature Sources

