Nicotinic acid inhibits glioma invasion by facilitating Snail1 degradation
- PMID: 28256591
- PMCID: PMC5335718
- DOI: 10.1038/srep43173
Nicotinic acid inhibits glioma invasion by facilitating Snail1 degradation
Abstract
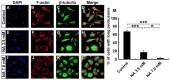
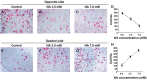
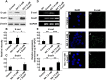
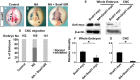
Malignant glioma is a formidable disease that commonly leads to death, mainly due to the invasion of tumor cells into neighboring tissues. Therefore, inhibition of tumor cell invasion may provide an effective therapy for malignant glioma. Here we report that nicotinic acid (NA), an essential vitamin, inhibits glioma cell invasion in vitro and in vivo. Treatment of the U251 glioma cells with NA in vitro results in reduced invasion, which is accompanied by a loss of mesenchymal phenotype and an increase in cell-cell adhesion. At the molecular level, transcription of the adherens junction protein E-cadherin is upregulated, leading to accumulation of E-cadherin protein at the cell-cell boundary. This can be attributed to NA's ability to facilitate the ubiquitination and degradation of Snail1, a transcription factor that represses E-cadherin expression. Similarly, NA transiently inhibits neural crest migration in Xenopus embryos in a Snail1-dependent manner, indicating that the mechanism of action for NA in cell migration is evolutionarily conserved. We further show that NA injection blocks the infiltration of tumor cells into the adjacent brain tissues and improves animal survival in a rat model of glioma. These results suggest that NA treatment may be developed into a potential therapy for malignant glioma.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Dandy W. E. Removal of right cerebral hemisphere for certain tumors with hemiplegia: Preliminary report. Journal of the American Medical Association 90, 823–825 (1928).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

