3D Printed Graphene Based Energy Storage Devices
- PMID: 28256602
- PMCID: PMC5361393
- DOI: 10.1038/srep42233
3D Printed Graphene Based Energy Storage Devices
Abstract
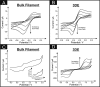
3D printing technology provides a unique platform for rapid prototyping of numerous applications due to its ability to produce low cost 3D printed platforms. Herein, a graphene-based polylactic acid filament (graphene/PLA) has been 3D printed to fabricate a range of 3D disc electrode (3DE) configurations using a conventional RepRap fused deposition moulding (FDM) 3D printer, which requires no further modification/ex-situ curing step. To provide proof-of-concept, these 3D printed electrode architectures are characterised both electrochemically and physicochemically and are advantageously applied as freestanding anodes within Li-ion batteries and as solid-state supercapacitors. These freestanding anodes neglect the requirement for a current collector, thus offering a simplistic and cheaper alternative to traditional Li-ion based setups. Additionally, the ability of these devices' to electrochemically produce hydrogen via the hydrogen evolution reaction (HER) as an alternative to currently utilised platinum based electrodes (with in electrolysers) is also performed. The 3DE demonstrates an unexpectedly high catalytic activity towards the HER (-0.46 V vs. SCE) upon the 1000th cycle, such potential is the closest observed to the desired value of platinum at (-0.25 V vs. SCE). We subsequently suggest that 3D printing of graphene-based conductive filaments allows for the simple fabrication of energy storage devices with bespoke and conceptual designs to be realised.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Brownson D. A. C. et al.. In situ electrochemical characterisation of graphene and various carbon-based electrode materials: an internal standard approach. RSC Adv. 5, 37281–37286 (2015).
-
- Li W. et al.. Electrochemistry of Individual Monolayer Graphene Sheets. ACS Nano 5, 2264–2270 (2011). - PubMed
-
- Huang H. & Zhu J.-J. The electrochemical applications of quantum dots. Analyst 138, 5855–5865 (2013). - PubMed
-
- Wang Y. et al.. All-Inorganic Colloidal Perovskite Quantum Dots: A New Class of Lasing Materials with Favorable Characteristics. Adv. Mat. 27, 7101–7108 (2015). - PubMed
-
- Burch H. A. et al.. Electrocatalytic regeneration of atmospherically aged MoS2 nanostructures via solution-phase sulfidation. RSC Adv. 6, 26689–26695 (2016).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

