The Rotavirus NSP4 Viroporin Domain is a Calcium-conducting Ion Channel
- PMID: 28256607
- PMCID: PMC5335360
- DOI: 10.1038/srep43487
The Rotavirus NSP4 Viroporin Domain is a Calcium-conducting Ion Channel
Abstract
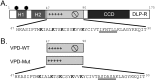
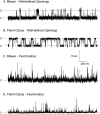
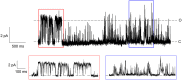
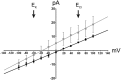
Viroporins are small virus-encoded ion channel proteins. Most viroporins are monovalent selective cation channels, with few showing the ability to conduct divalent cations, like calcium (Ca2+). Nevertheless, some viroporins are known to disrupt host cell Ca2+ homeostasis, which is critical for virus replication and pathogenesis. Rotavirus nonstructural protein 4 (NSP4) is an endoplasmic reticulum transmembrane glycoprotein that has a viroporin domain (VPD), and NSP4 viroporin activity elevates cytosolic Ca2+ in mammalian cells. The goal of this study was to demonstrate that the NSP4 VPD forms an ion channel and determine whether the channel can conduct Ca2+. Using planar lipid bilayer and liposome patch clamp electrophysiology, we show that a synthetic peptide of the NSP4 VPD has ion channel activity. The NSP4 VPD was selective for cations over anions and channel activity was observed to have both well-defined "square top" openings as well as fast current fluctuations, similar to other viroporins. Importantly, the NSP4 VPD showed similar conductance of divalent cations (Ca2+ and Ba2+) as monovalent cations (K+), but a viroporin defective mutant lacked Ca2+ conductivity. These data demonstrate that the NSP4 VPD is a Ca2+-conducting viroporin and establish the mechanism by which NSP4 disturbs host cell Ca2+ homeostasis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity.mBio. 2010 Nov 30;1(5):e00265-10. doi: 10.1128/mBio.00265-10. mBio. 2010. PMID: 21151776 Free PMC article.
-
Activation of the endoplasmic reticulum calcium sensor STIM1 and store-operated calcium entry by rotavirus requires NSP4 viroporin activity.J Virol. 2013 Dec;87(24):13579-88. doi: 10.1128/JVI.02629-13. Epub 2013 Oct 9. J Virol. 2013. PMID: 24109210 Free PMC article.
-
Genetic divergence of rotavirus nonstructural protein 4 results in distinct serogroup-specific viroporin activity and intracellular punctate structure morphologies.J Virol. 2012 May;86(9):4921-34. doi: 10.1128/JVI.06759-11. Epub 2012 Feb 22. J Virol. 2012. PMID: 22357281 Free PMC article.
-
Rotavirus protein expression is important for virus assembly and pathogenesis.Arch Virol Suppl. 1996;12:69-77. doi: 10.1007/978-3-7091-6553-9_8. Arch Virol Suppl. 1996. PMID: 9015103 Review.
-
Rotavirus NSP4: a multifunctional viral enterotoxin.Viral Immunol. 2005;18(1):27-40. doi: 10.1089/vim.2005.18.27. Viral Immunol. 2005. PMID: 15802952 Review. No abstract available.
Cited by
-
Beyond Channel Activity: Protein-Protein Interactions Involving Viroporins.Subcell Biochem. 2018;88:329-377. doi: 10.1007/978-981-10-8456-0_15. Subcell Biochem. 2018. PMID: 29900504 Free PMC article. Review.
-
Equine Rotavirus A under the One Health Lens: Potential Impacts on Public Health.Viruses. 2024 Jan 16;16(1):130. doi: 10.3390/v16010130. Viruses. 2024. PMID: 38257830 Free PMC article. Review.
-
Screening of candidate genes associated with high titer production of oncolytic measles virus based on systems biology approach.Virus Genes. 2022 Aug;58(4):270-283. doi: 10.1007/s11262-022-01902-y. Epub 2022 Apr 27. Virus Genes. 2022. PMID: 35477822
-
Treading a HOSTile path: Mapping the dynamic landscape of host cell-rotavirus interactions to explore novel host-directed curative dimensions.Virulence. 2021 Dec;12(1):1022-1062. doi: 10.1080/21505594.2021.1903198. Virulence. 2021. PMID: 33818275 Free PMC article. Review.
-
SARS-COV-2, infection, transmission, transcription, translation, proteins, and treatment: A review.Int J Biol Macromol. 2021 Dec 15;193(Pt B):1249-1273. doi: 10.1016/j.ijbiomac.2021.10.172. Epub 2021 Oct 28. Int J Biol Macromol. 2021. PMID: 34756970 Free PMC article. Review.
References
-
- Scott C. & Griffin S. Viroporins: structure, function and potential as antiviral targets. J Gen. Virol. 96, 2000–2027 (2015). - PubMed
-
- Hyser J. M. Viroporins in Electrophysiology of Unconventional Channels and Pores(ed. Delcour A. H.) 153–181 (Springer, Switzerland, 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

