Pre-Exposure Gene Expression in Baboons with and without Pancytopenia after Radiation Exposure
- PMID: 28257102
- PMCID: PMC5372557
- DOI: 10.3390/ijms18030541
Pre-Exposure Gene Expression in Baboons with and without Pancytopenia after Radiation Exposure
Abstract
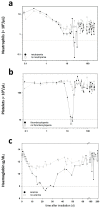
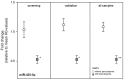
Radiosensitivity differs in humans and likely among primates. The reasons are not well known. We examined pre-exposure gene expression in baboons (n = 17) who developed haematologic acute radiation syndrome (HARS) without pancytopenia or a more aggravated HARS with pancytopenia after irradiation. We evaluated gene expression in a two stage study design where stage I comprised a whole genome screen for messenger RNAs (mRNA) (microarray) and detection of 667 microRNAs (miRNA) (real-time quantitative polymerase chain reaction (qRT-PCR) platform). Twenty candidate mRNAs and nine miRNAs were selected for validation in stage II (qRT-PCR). None of the mRNA species could be confirmed during the validation step, but six of the nine selected candidate miRNA remained significantly different during validation. In particular, miR-425-5p (receiver operating characteristic = 0.98; p = 0.0003) showed nearly complete discrimination between HARS groups with and without pancytopenia. Target gene searches of miR-425-5p identified new potential mRNAs and associated biological processes linked with radiosensitivity. We found that one miRNA species examined in pre-exposure blood samples was associated with HARS characterized by pancytopenia and identified new target mRNAs that might reflect differences in radiosensitivity of irradiated normal tissue.
Keywords: gene expression; haematologic acute radiation syndrome (HARS); miRNA; pancytopenia; radiosensitivity.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Gene expression signature for early prediction of late occurring pancytopenia in irradiated baboons.Ann Hematol. 2017 May;96(5):859-870. doi: 10.1007/s00277-017-2952-7. Epub 2017 Feb 24. Ann Hematol. 2017. PMID: 28236054 Free PMC article.
-
MicroRNA Expression for Early Prediction of Late Occurring Hematologic Acute Radiation Syndrome in Baboons.PLoS One. 2016 Nov 15;11(11):e0165307. doi: 10.1371/journal.pone.0165307. eCollection 2016. PLoS One. 2016. PMID: 27846229 Free PMC article.
-
First Generation Gene Expression Signature for Early Prediction of Late Occurring Hematological Acute Radiation Syndrome in Baboons.Radiat Res. 2016 Jul;186(1):39-54. doi: 10.1667/RR14318.1. Epub 2016 Jun 22. Radiat Res. 2016. PMID: 27333084
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Gene Expression Changes in Irradiated Baboons: A Summary and Interpretation of a Decade of Findings.Radiat Res. 2021 Jun 1;195(6):501-521. doi: 10.1667/RADE-20-00217.1. Radiat Res. 2021. PMID: 33788952 Review. No abstract available.
Cited by
-
Transcriptome of rhesus macaque (Macaca mulatta) exposed to total-body irradiation.Sci Rep. 2021 Mar 18;11(1):6295. doi: 10.1038/s41598-021-85669-6. Sci Rep. 2021. PMID: 33737626 Free PMC article.
-
Development of Biomarkers for Radiation Biodosimetry and Medical Countermeasures Research: Current Status, Utility, and Regulatory Pathways.Radiat Res. 2022 May 1;197(5):514-532. doi: 10.1667/RADE-21-00157.1. Radiat Res. 2022. PMID: 34879151 Free PMC article.
-
Persistent mRNA and miRNA expression changes in irradiated baboons.Sci Rep. 2018 Oct 18;8(1):15353. doi: 10.1038/s41598-018-33544-2. Sci Rep. 2018. PMID: 30337559 Free PMC article.
-
A Review of Radiation-Induced Alterations of Multi-Omic Profiles, Radiation Injury Biomarkers, and Countermeasures.Radiat Res. 2023 Jan 1;199(1):89-111. doi: 10.1667/RADE-21-00187.1. Radiat Res. 2023. PMID: 36368026 Free PMC article. Review.
-
Nonhuman primates as models for the discovery and development of radiation countermeasures.Expert Opin Drug Discov. 2017 Jul;12(7):695-709. doi: 10.1080/17460441.2017.1323863. Epub 2017 May 5. Expert Opin Drug Discov. 2017. PMID: 28441902 Free PMC article. Review.
References
-
- International Atomic Energy Agency . Cytogenetic Analysis for Radiation Dose Assessment: A Manual. International Atomic Energy Agency; Wien, Austria: 2001.
-
- Farese A.M., MacVittie T.J. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today. 2015;51:537–548. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

