New Cytotoxic Seco-Type Triterpene and Labdane-Type Diterpenes from Nuxia oppositifolia
- PMID: 28257105
- PMCID: PMC6155346
- DOI: 10.3390/molecules22030389
New Cytotoxic Seco-Type Triterpene and Labdane-Type Diterpenes from Nuxia oppositifolia
Abstract
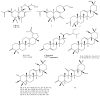
Chromatographic purification of the n-hexane and dichloromethane extracts of Nuxia oppositifolia aerial parts, growing in Saudi Arabia, resulted in the isolation and characterization of three new labdane-type diterpene acids, 2β-acetoxy-labda-7-en-15-oic acid (1), 2β-acetoxy-7-oxolabda-8-en-15-oic acid (2), 2β-acetoxy-6-oxolabda-7-en-15-oic acid (3), and one new seco-triterpene, 3,4-seco olean-12-en-3,30 dioic acid (4), together with 10 known lupane, oleanane and ursane-type triterpenes, as well as the common phytosterols, β-sitosterol and stigmasterol (5-16). Their structures have been assigned on the basis of different spectroscopic techniques including 1D and 2D NMR. Moreover, 13 of the isolated compounds were tested on the human cancer cell lines HeLa (cervical), A549 (lung) and MDA (breast), and most of the compounds showed potent cytotoxic activities in vitro.
Keywords: Nuxia oppositifolia; cytotoxicity; labdane-type diterpene; seco-triterpene; triterpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cytotoxic lupane-, secolupane-, and oleanane-type triterpenes from Viburnum awabuki.Nat Prod Res. 2008 Feb 15;22(3):191-7. doi: 10.1080/14786410701761019. Nat Prod Res. 2008. PMID: 18266145
-
Two new diterpene derivatives from Euphorbia lunulata Bge and their anti-proliferative activities.Fitoterapia. 2014 Jul;96:33-8. doi: 10.1016/j.fitote.2014.03.016. Epub 2014 Mar 29. Fitoterapia. 2014. PMID: 24685501
-
Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds.Molecules. 2019 Nov 9;24(22):4060. doi: 10.3390/molecules24224060. Molecules. 2019. PMID: 31717557 Free PMC article.
-
Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy.Planta Med. 2009 Dec;75(15):1549-60. doi: 10.1055/s-0029-1186102. Planta Med. 2009. PMID: 19742422 Review.
-
Plant-derived terpenoids and analogues as anti-HIV agents.Curr Top Med Chem. 2003;3(2):155-69. doi: 10.2174/1568026033392435. Curr Top Med Chem. 2003. PMID: 12570771 Review.
Cited by
-
Cell proliferation activity delineated by molecular docking of four new compounds isolated from the aerial parts of Suaeda monoica Forssk. ex. J.F. Gmel.Saudi Pharm J. 2020 Feb;28(2):172-186. doi: 10.1016/j.jsps.2019.11.019. Epub 2019 Dec 7. Saudi Pharm J. 2020. PMID: 32042256 Free PMC article.
-
Unveiling the pharmacological potential of plant triterpenoids in breast cancer management: an updated review.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):5571-5596. doi: 10.1007/s00210-024-03054-2. Epub 2024 Apr 2. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38563878 Free PMC article. Review.
-
Insecticidal Activity and Free Radical Scavenging Properties of Isolated Phytoconstituents from the Saudi Plant Nuxia oppositifolia (Hochst.).Molecules. 2021 Feb 9;26(4):914. doi: 10.3390/molecules26040914. Molecules. 2021. PMID: 33572261 Free PMC article.
-
Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia.Biomolecules. 2019 Dec 30;10(1):61. doi: 10.3390/biom10010061. Biomolecules. 2019. PMID: 31905962 Free PMC article.
-
Evaluation of Antimalarial Activity of Hydromethanolic Crude Extract and Solvent Fractions of the Leaves of Nuxia congesta R. Br. Ex Fresen (Buddlejaceae) in Plasmodium berghei Infected Mice.J Exp Pharmacol. 2019 Dec 16;11:121-134. doi: 10.2147/JEP.S230636. eCollection 2019. J Exp Pharmacol. 2019. PMID: 31908546 Free PMC article.
References
-
- Johnson L. Review of the systematics of scrophulariaceae s.L. And their current disposition. Aust. Syst. Bot. 2006;19:289–307.
-
- Al-Abbasi T.M., Al-Farhan A.H., Al-Khulaidi W., Hall M., Lewellyn O.A., Miller A.G., Patzelt A. Important plant areas in the Arabian peninsula. Edinb. J. Bot. 2010;67:25–35. doi: 10.1017/S0960428609990217. - DOI
-
- Migahid A.M. Flora of Saudi Arabia. 3rd ed. Volume II University Libraries, King Saud University; Riyadh, Saudi Arabia: 1989.
-
- Jonville M.C., Kodja H., Strasberg D., Pichette A., Ollivier E., Frederich M., Angenot L., Legault J. Antiplasmodial, anti-inflammatory and cytotoxic activities of various plant extracts from the Mascarene archipelago. J. Ethnopharmacol. 2011;136:525–531. doi: 10.1016/j.jep.2010.06.013. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials