Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation
- PMID: 28257428
- PMCID: PMC5336265
- DOI: 10.1371/journal.pone.0173174
Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation
Abstract
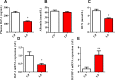
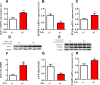
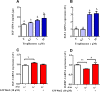
Dietary protein or amino acid (AA) is a crucial nutritional factor to regulate hepatic insulin-like growth factor-1 (IGF-1) expression and secretion. However, the underlying intracellular mechanism by which dietary protein or AA induces IGF-1 expression remains unknown. We compared the IGF-1 gene expression and plasma IGF-1 level of pigs fed with normal crude protein (CP, 20%) and low-protein levels (LP, 14%). RNA sequencing (RNA-seq) was performed to detect transcript expression in the liver in response to dietary protein. The results showed that serum concentrations and mRNA levels of IGF-1 in the liver were higher in the CP group than in the LP group. RNA-seq analysis identified a total of 1319 differentially expressed transcripts (667 upregulated and 652 downregulated), among which the terms "oxidative phosphorylation", "ribosome", "gap junction", "PPAR signaling pathway", and "focal adhesion" were enriched. In addition, the porcine primary hepatocyte and HepG2 cell models also demonstrated that the mRNA and protein levels of IGF-1 and PPARγ increased with the increasing AA concentration in the culture. The PPARγ activator troglitazone increased IGF-1 gene expression and secretion in a dose dependent manner. Furthermore, inhibition of PPARγ effectively reversed the effects of the high AA concentration on the mRNA expression of IGF-1 and IGFBP-1 in HepG2 cells. Moreover, the protein levels of IGF-1 and PPARγ, as well as the phosphorylation of mTOR, significantly increased in HepG2 cells under high AA concentrations. mTOR phosphorylation can be decreased by the mTOR antagonist, rapamycin. The immunoprecipitation results also showed that high AA concentrations significantly increased the interaction of mTOR and PPARγ. In summary, PPARγ plays an important role in the regulation of IGF-1 secretion and gene expression in response to dietary protein.
Conflict of interest statement
Figures



References
-
- Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. The Journal of endocrinology. 2001;170(1):63–70. Epub 2001/06/30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

