Silk-Derived Protein Enhances Corneal Epithelial Migration, Adhesion, and Proliferation
- PMID: 28257533
- PMCID: PMC6022413
- DOI: 10.1167/iovs.16-19957
Silk-Derived Protein Enhances Corneal Epithelial Migration, Adhesion, and Proliferation
Abstract
Purpose: The corneal surface is vulnerable to a myriad of traumatic insults including mechanical, chemical, and thermal injuries. The resulting trauma may render the naturally occurring regenerative properties of the cornea incapable of restoring a healthy epithelial surface, and may result in the loss of corneal transparency and vision. Healing of the corneal epithelium requires a complex cascade of biological processes that work to restore the tissue after injury. New therapeutic agents that act on the multiple steps of the corneal wound-healing process would offer a potential for improving patient outcomes. Here, a novel silk fibroin-derived protein (SDP) was studied for potential impacts on wound healing through studying an in vitro model.
Methods: Solubilized SDP, produced from the Bombyx mori silkworm cocoon, was added to human corneal limbal-epithelial (hCLE) cultures to evaluate the material's effects on epithelial cell migration, proliferation, and adhesion through the use of various scratch wound assays and flow chamber studies.
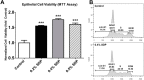
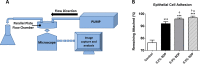
Results: Results indicated that the addition of SDP to culture increased hCLE migration rate by over 50%, and produced an approximate 60% increase in cell proliferation. This resulted in a nearly 30% enhancement of in vitro scratch wound closure time. In addition, cultures treated with SDP experienced increased cell-matrix focal adhesion formation by over 95% when compared to controls.
Conclusions: The addition of SDP to culture media significantly enhanced hCLE cell sheet migration, proliferation, and attachment when compared to untreated controls, and indicates SDP's potential utility as an ophthalmic therapeutic agent.
Figures




References
-
- Agrawal VB, Tsai RJ. . Corneal epithelial wound healing. Indian J Ophthalmol. 2003; 51: 5– 15. - PubMed
-
- Yiu S, Thomas P, Nguyen P. . Ocular surface reconstruction: recent advances and future outlook. Curr Opin Ophthalmol. 2007; 18: 509– 514. - PubMed
-
- Suzuki K. . Cell–matrix and cell–cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003; 22: 113– 133. - PubMed
-
- Boulton M, Albon J. . Stem cells in the eye. Int J Biochem Cell Biol. 2004; 36: 643– 657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

