Human Mesenchymal Stem Cell-Educated Macrophages Are a Distinct High IL-6-Producing Subset that Confer Protection in Graft-versus-Host-Disease and Radiation Injury Models
- PMID: 28257800
- PMCID: PMC5499382
- DOI: 10.1016/j.bbmt.2017.02.018
Human Mesenchymal Stem Cell-Educated Macrophages Are a Distinct High IL-6-Producing Subset that Confer Protection in Graft-versus-Host-Disease and Radiation Injury Models
Abstract
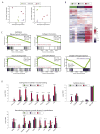
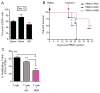
Mesenchymal stem cells (MSCs) have immunosuppressive and tissue repair properties, but clinical trials using MSCs to prevent or treat graft-versus-host disease (GVHD) have shown mixed results. Macrophages (MØs) are important regulators of immunity and can promote tissue regeneration and remodeling. We have previously shown that MSCs can educate MØs toward a unique anti-inflammatory immunophenotype (MSC-educated MØs [MEMs]); however, their implications for in vivo models of inflammation have not been studied yet. We now show that in comparison with MØs, MEMs have increased expression of the inhibitory molecules PD-L1, PD-L2, in addition to markers of alternatively activated MØs: CD206 and CD163. RNA-Seq analysis of MEMs, as compared with MØs, show a distinct gene expression profile that positively correlates with multiple pathways important in tissue repair. MEMs also show increased expression of IL-6, transforming growth factor-β, arginase-1, CD73, and decreased expression of IL-12 and tumor necrosis factor-α. We show that IL-6 secretion is controlled in part by the cyclo-oxygenase-2, arginase, and JAK1/STAT1 pathway. When tested in vivo, we show that human MEMs significantly enhance survival from lethal GVHD and improve survival of mice from radiation injury. We show these effects could be mediated in part through suppression of human T cell proliferation and may have attenuated host tissue injury in part by enhancing murine fibroblast proliferation. MEMs are a unique MØ subset with therapeutic potential for the management of GVHD and/or protection from radiation-induced injury.
Keywords: Graft-versus-host disease (GVHD); IL-6; MSC-educated macrophages (MEMs); Macrophages; Mesenchymal stem cells (MSCs); Radiation injury.
Copyright © 2017 The American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Figures

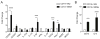
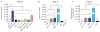
Comment in
-
Commentary: Role of Mesenchymal Stromal Cell-Mediated Crosstalk with Macrophages in Graft-versus-Host Disease and Tissue Repair.Biol Blood Marrow Transplant. 2017 Jun;23(6):861-862. doi: 10.1016/j.bbmt.2017.04.006. Epub 2017 Apr 7. Biol Blood Marrow Transplant. 2017. PMID: 28396165 No abstract available.
References
-
- Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–644. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

