Dynamic Notch Signaling Specifies Each Cell Fate in Drosophila Spermathecal Lineage
- PMID: 28258114
- PMCID: PMC5427495
- DOI: 10.1534/g3.117.040212
Dynamic Notch Signaling Specifies Each Cell Fate in Drosophila Spermathecal Lineage
Abstract
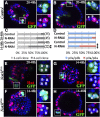
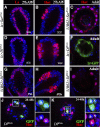
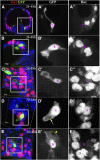
Spermathecae are glandular organs in the insect female reproductive tract that play essential roles in insect reproduction; however, the molecular mechanism involved in their development is largely unknown. Drosophila spermathecae consist of class-III secretory units, in which each secretory cell (SC) discharges its products to the central lumen through an end-apparatus and a canal. Secretory unit formation in Drosophila spermathecae utilizes a fixed cell lineage, in which each secretory unit precursor (SUP) divides to produce one pIIb cell and one pIIa cell. The former differentiates into an apical cell (AC), whereas the latter divides again to produce an SC and a basal cell (BC). It is unclear how each cell acquires its identity and contributes to secretory unit formation. Here, we demonstrate that Notch signaling is required and sufficient for the specification of lumen epithelial precursors (LEPs; vs. SUPs), pIIb (vs. pIIa), and SCs (vs. BCs) sequentially. To our surprise, Notch activation in LEPs and SCs apparently utilizes different ligand mechanisms. In addition, Notch signaling both suppresses and activates transcription factors Hindsight (Hnt) and Cut during spermathecal lineage specification, supporting the notion that Notch signaling can have opposite biological outcomes in different cellular environments. Furthermore, LEP-derived epithelial cells (ECs) and ACs show distinct cellular morphology and are essential for securing secretory units to the epithelial lumen. Our work demonstrates, for the first time, the dynamic role of Notch signaling in binary cell fate determination in Drosophila spermathecae and the role of ECs and ACs in secretory unit formation.
Keywords: Cut; Notch signaling; binary cell fate determination; class-III secretory gland; spermathecae.
Copyright © 2017 Shen and Sun.
Figures

Similar articles
-
Different modes of Notch activation and strength regulation in the spermathecal secretory lineage.Development. 2020 Feb 7;147(3):dev184390. doi: 10.1242/dev.184390. Development. 2020. PMID: 31988187 Free PMC article.
-
Intra-lineage Fate Decisions Involve Activation of Notch Receptors Basal to the Midbody in Drosophila Sensory Organ Precursor Cells.Curr Biol. 2017 Aug 7;27(15):2239-2247.e3. doi: 10.1016/j.cub.2017.06.030. Epub 2017 Jul 20. Curr Biol. 2017. PMID: 28736165
-
Par3 cooperates with Sanpodo for the assembly of Notch clusters following asymmetric division of Drosophila sensory organ precursor cells.Elife. 2021 Oct 1;10:e66659. doi: 10.7554/eLife.66659. Elife. 2021. PMID: 34596529 Free PMC article.
-
Notch signaling and the generation of cell diversity in Drosophila neuroblast lineages.Adv Exp Med Biol. 2012;727:47-60. doi: 10.1007/978-1-4614-0899-4_4. Adv Exp Med Biol. 2012. PMID: 22399338 Review.
-
Notch signaling in the pancreas: patterning and cell fate specification.Wiley Interdiscip Rev Dev Biol. 2013 Jul;2(4):531-44. doi: 10.1002/wdev.99. Epub 2012 Dec 6. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24014421 Review.
Cited by
-
Polyploidy in development and tumor models in Drosophila.Semin Cancer Biol. 2022 Jun;81:106-118. doi: 10.1016/j.semcancer.2021.09.011. Epub 2021 Sep 22. Semin Cancer Biol. 2022. PMID: 34562587 Free PMC article. Review.
-
A Functional Analysis of the Drosophila Gene hindsight: Evidence for Positive Regulation of EGFR Signaling.G3 (Bethesda). 2020 Jan 7;10(1):117-127. doi: 10.1534/g3.119.400829. G3 (Bethesda). 2020. PMID: 31649045 Free PMC article.
-
Regional specialization, polyploidy, and seminal fluid transcripts in the Drosophila female reproductive tract.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2409850121. doi: 10.1073/pnas.2409850121. Epub 2024 Oct 25. Proc Natl Acad Sci U S A. 2024. PMID: 39453739 Free PMC article.
-
Finishing the egg.Genetics. 2024 Jan 3;226(1):iyad183. doi: 10.1093/genetics/iyad183. Genetics. 2024. PMID: 38000906 Free PMC article.
-
Different modes of Notch activation and strength regulation in the spermathecal secretory lineage.Development. 2020 Feb 7;147(3):dev184390. doi: 10.1242/dev.184390. Development. 2020. PMID: 31988187 Free PMC article.
References
-
- Allen A. K., Spradling A. C., 2008. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 135: 311–321. - PubMed
-
- Artavanis-Tsakonas S., Rand M. D., Lake R. J., 1999. Notch signaling: cell fate control and signal integration in development. Science 284: 770–776. - PubMed
-
- Bhat K. M., 2014. Notch signaling acts before cell division to promote asymmetric cleavage and cell fate of neural precursor cells. Sci. Signal. 7: ra101. - PubMed
-
- Billen J., 2011. Exocrine glands and their key function in the communication system of social insects. Formos. Entomol 31: 75–84.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
