Rationalized design of a mucosal vaccine protects against Mycobacterium tuberculosis challenge in mice
- PMID: 28258153
- PMCID: PMC5433857
- DOI: 10.1189/jlb.4A0616-270R
Rationalized design of a mucosal vaccine protects against Mycobacterium tuberculosis challenge in mice
Abstract
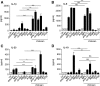
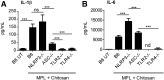
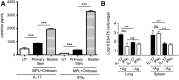
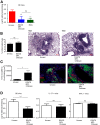
Pulmonary tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) is a leading cause of global morbidity and mortality. The only licensed TB vaccine, Mycobacterium bovis bacillus Calmette-Guerin (BCG), has variable efficacy in protecting against pulmonary TB. Thus, the development of more effective TB vaccines is critical to control the TB epidemic. Specifically, vaccines delivered through the mucosal route are known to induce Th17 responses and provide superior protection against Mtb infection. However, already tested Th17-inducing mucosal adjuvants, such as heat-labile enterotoxins and cholera toxins, are not considered safe for use in humans. In the current study, we rationally screened adjuvants for their ability to induce Th17-polarizing cytokines in dendritic cells (DCs) and determined whether they could be used in a protective mucosal TB vaccine. Our new studies show that monophosphoryl lipid A (MPL), when used in combination with chitosan, potently induces Th17-polarizing cytokines in DCs and downstream Th17/Th1 mucosal responses and confers significant protection in mice challenged with a clinical Mtb strain. Additionally, we show that both TLRs and the inflammasome pathways are activated in DCs by MPL-chitosan to mediate induction of Th17-polarizing cytokines. Together, our studies put forward the potential of a new, protective mucosal TB vaccine candidate, which incorporates safe adjuvants already approved for use in humans.
Keywords: Th17/Th1 responses; adjuvants; mucosal immunity; tuberculosis.
© Society for Leukocyte Biology.
Figures
References
-
- World Health Organization (2015) Global Tuberculosis Report 2015 WHO, Geneva, Switzerland.
-
- Dye C., Glaziou P., Floyd K., Raviglione M. (2013) Prospects for tuberculosis elimination. Annu. Rev. Public Health 34, 271–286. - PubMed
-
- Goonetilleke N. P., McShane H., Hannan C. M., Anderson R. J., Brookes R. H., Hill A. V. (2003) Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171, 1602–1609. - PubMed
-
- Wang J., Thorson L., Stokes R. W., Santosuosso M., Huygen K., Zganiacz A., Hitt M., Xing Z. (2004) Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 173, 6357–6365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

