Inhibition of L-Type Ca2+ Channels by TRPC1-STIM1 Complex Is Essential for the Protection of Dopaminergic Neurons
- PMID: 28258168
- PMCID: PMC5373123
- DOI: 10.1523/JNEUROSCI.3010-16.2017
Inhibition of L-Type Ca2+ Channels by TRPC1-STIM1 Complex Is Essential for the Protection of Dopaminergic Neurons
Abstract
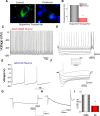
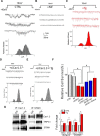
Loss of dopaminergic (DA) neurons leads to Parkinson's disease; however, the mechanism(s) for the vulnerability of DA neurons is(are) not fully understood. We demonstrate that TRPC1 regulates the L-type Ca2+ channel that contributes to the rhythmic activity of adult DA neurons in the substantia nigra region. Store depletion that activates TRPC1, via STIM1, inhibits the frequency and amplitude of the rhythmic activity in DA neurons of wild-type, but not in TRPC1-/-, mice. Similarly, TRPC1-/- substantia nigra neurons showed increased L-type Ca2+ currents, decreased stimulation-dependent STIM1-Cav1.3 interaction, and decreased DA neurons. L-type Ca2+ currents and the open channel probability of Cav1.3 channels were also reduced upon TRPC1 activation, whereas increased Cav1.3 currents were observed upon STIM1 or TRPC1 silencing. Increased interaction between Cav1.3-TRPC1-STIM1 was observed upon store depletion and the loss of either TRPC1 or STIM1 led to DA cell death, which was prevented by inhibiting L-type Ca2+ channels. Neurotoxins that mimic Parkinson's disease increased Cav1.3 function, decreased TRPC1 expression, inhibited Tg-mediated STIM1-Cav1.3 interaction, and induced caspase activation. Importantly, restoration of TRPC1 expression not only inhibited Cav1.3 function but increased cell survival. Together, we provide evidence that TRPC1 suppresses Cav1.3 activity by providing an STIM1-based scaffold, which is essential for DA neuron survival.SIGNIFICANCE STATEMENT Ca2+ entry serves critical cellular functions in virtually every cell type, and appropriate regulation of Ca2+ in neurons is essential for proper function. In Parkinson's disease, DA neurons are specifically degenerated, but the mechanism is not known. Unlike other neurons, DA neurons depend on Cav1.3 channels for their rhythmic activity. Our studies show that, in normal conditions, the pacemaking activity in DA neurons is inhibited by the TRPC1-STIM1 complex. Neurotoxins that mimic Parkinson's disease target TRPC1 expression, which leads to an abnormal increase in Cav1.3 activity, thereby causing degeneration of DA neurons. These findings link TRPC1 to Cav1.3 regulation and provide important indications about how disrupting Ca2+ balance could have a direct implication in the treatment of Parkinson's patients.
Keywords: Cav1.3; Parkinson's disease; SOCE; TRPC1-STIM1; calcium.
Copyright © 2017 the authors 0270-6474/17/373364-14$15.00/0.
Figures






Similar articles
-
Lower Affinity of Isradipine for L-Type Ca2+ Channels during Substantia Nigra Dopamine Neuron-Like Activity: Implications for Neuroprotection in Parkinson's Disease.J Neurosci. 2017 Jul 12;37(28):6761-6777. doi: 10.1523/JNEUROSCI.2946-16.2017. Epub 2017 Jun 7. J Neurosci. 2017. PMID: 28592699 Free PMC article.
-
Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons.Brain. 2014 Aug;137(Pt 8):2287-302. doi: 10.1093/brain/awu131. Epub 2014 Jun 16. Brain. 2014. PMID: 24934288 Free PMC article.
-
Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling.J Clin Invest. 2012 Apr;122(4):1354-67. doi: 10.1172/JCI61332. Epub 2012 Mar 26. J Clin Invest. 2012. PMID: 22446186 Free PMC article.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
-
TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces.Cell Calcium. 2017 May;63:33-39. doi: 10.1016/j.ceca.2016.12.009. Epub 2016 Dec 30. Cell Calcium. 2017. PMID: 28089266 Free PMC article. Review.
Cited by
-
The Role of Calcium and Iron Homeostasis in Parkinson's Disease.Brain Sci. 2024 Jan 17;14(1):88. doi: 10.3390/brainsci14010088. Brain Sci. 2024. PMID: 38248303 Free PMC article. Review.
-
Deletion of Stim1 in Hypothalamic Arcuate Nucleus Kiss1 Neurons Potentiates Synchronous GCaMP Activity and Protects against Diet-Induced Obesity.J Neurosci. 2021 Nov 24;41(47):9688-9701. doi: 10.1523/JNEUROSCI.0622-21.2021. Epub 2021 Oct 15. J Neurosci. 2021. PMID: 34654752 Free PMC article.
-
Molecular physiology and pathophysiology of stromal interaction molecules.Exp Biol Med (Maywood). 2018 Mar;243(5):451-472. doi: 10.1177/1535370218754524. Epub 2018 Jan 24. Exp Biol Med (Maywood). 2018. PMID: 29363328 Free PMC article. Review.
-
Target Molecules of STIM Proteins in the Central Nervous System.Front Mol Neurosci. 2020 Dec 23;13:617422. doi: 10.3389/fnmol.2020.617422. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33424550 Free PMC article. Review.
-
STIM Proteins: The Gas and Brake of Calcium Entry in Neurons.Neurosci Bull. 2025 Feb;41(2):305-325. doi: 10.1007/s12264-024-01272-5. Epub 2024 Sep 12. Neurosci Bull. 2025. PMID: 39266936 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
