Structural Implications for the Formation and Function of the Complement Effector Protein iC3b
- PMID: 28258193
- PMCID: PMC5478675
- DOI: 10.4049/jimmunol.1601864
Structural Implications for the Formation and Function of the Complement Effector Protein iC3b
Abstract
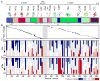
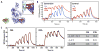
Complement-mediated opsonization, phagocytosis, and immune stimulation are critical processes in host defense and homeostasis, with the complement activation fragment iC3b playing a key effector role. To date, however, there is no high-resolution structure of iC3b, and some aspects of its structure-activity profile remain controversial. Here, we employed hydrogen-deuterium exchange mass spectrometry to describe the structure and dynamics of iC3b at a peptide resolution level in direct comparison with its parent protein C3b. In our hydrogen-deuterium exchange mass spectrometry study, 264 peptides were analyzed for their deuterium content, providing almost complete sequence coverage for this 173-kDa protein. Several peptides in iC3b showed significantly higher deuterium uptake when compared with C3b, revealing more dynamic, solvent-exposed regions. Most of them resided in the CUB domain, which contains the heptadecapeptide C3f that is liberated during the conversion of C3b to iC3b. Our data suggest a highly disordered CUB, which has acquired a state similar to that of intrinsically disordered proteins, resulting in a predominant form of iC3b that features high structural flexibility. The structure was further validated using an anti-iC3b mAb that was shown to target an epitope in the CUB region. The information obtained in this work allows us to elucidate determinants of iC3b specificity and activity and provide functional insights into the protein's recognition pattern with respect to regulators and receptors of the complement system.
Copyright © 2017 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors state no conflict of interest.
Figures
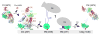

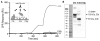

References
-
- Janssen BJC, Huizinga EG, Raaijmakers HCA, Roos A, Daha MR, Nilsson-Ekdahl K, Nilsson B, Gros P. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437:505–511. - PubMed
-
- Janssen BJC, Christodoulidou A, McCarthy A, Lambris JD, Gros P. Structure of C3b reveals conformational changes that underlie complement activity. Nature. 2006;444:213–216. - PubMed
-
- Roozendaal R, Carroll MC. Complement receptors CD21 and CD35 in humoral immunity. Immunol Rev. 2007;219:157–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

