Novel Adjuvant Based on the Pore-Forming Protein Sticholysin II Encapsulated into Liposomes Effectively Enhances the Antigen-Specific CTL-Mediated Immune Response
- PMID: 28258198
- PMCID: PMC5686032
- DOI: 10.4049/jimmunol.1600310
Novel Adjuvant Based on the Pore-Forming Protein Sticholysin II Encapsulated into Liposomes Effectively Enhances the Antigen-Specific CTL-Mediated Immune Response
Abstract
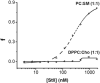
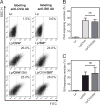
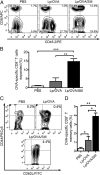
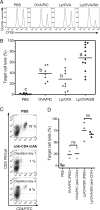
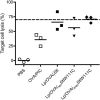
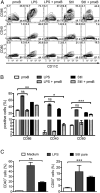
Vaccine strategies to enhance CD8+ CTL responses remain a current challenge because they should overcome the plasmatic and endosomal membranes for favoring exogenous Ag access to the cytosol of APCs. As a way to avoid this hurdle, sticholysin (St) II, a pore-forming protein from the Caribbean Sea anemone Stichodactyla helianthus, was encapsulated with OVA into liposomes (Lp/OVA/StII) to assess their efficacy to induce a CTL response. OVA-specific CD8+ T cells transferred to mice immunized with Lp/OVA/StII experienced a greater expansion than when the recipients were injected with the vesicles without St, mostly exhibiting a memory phenotype. Consequently, Lp/OVA/StII induced a more potent effector function, as shown by CTLs, in vivo assays. Furthermore, treatment of E.G7-OVA tumor-bearing mice with Lp/OVA/StII significantly reduced tumor growth being more noticeable in the preventive assay. The contribution of CD4+ and CD8+ T cells to CTL and antitumor activity, respectively, was elucidated. Interestingly, the irreversibly inactive variant of the StI mutant StI W111C, encapsulated with OVA into Lp, elicited a similar OVA-specific CTL response to that observed with Lp/OVA/StII or vesicles encapsulating recombinant StI or the reversibly inactive StI W111C dimer. These findings suggest the relative independence between StII pore-forming activity and its immunomodulatory properties. In addition, StII-induced in vitro maturation of dendritic cells might be supporting these properties. These results are the first evidence, to our knowledge, that StII, a pore-forming protein from a marine eukaryotic organism, encapsulated into Lp functions as an adjuvant to induce a robust specific CTL response.
Copyright © 2017 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

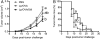
Similar articles
-
The Vacuolar Pathway in Macrophages Plays a Major Role in Antigen Cross-Presentation Induced by the Pore-Forming Protein Sticholysin II Encapsulated Into Liposomes.Front Immunol. 2018 Nov 5;9:2473. doi: 10.3389/fimmu.2018.02473. eCollection 2018. Front Immunol. 2018. PMID: 30455685 Free PMC article.
-
Sticholysin II shows similar immunostimulatory properties to LLO stimulating dendritic cells and MHC-I restricted T cell responses of heterologous antigen.Toxicon. 2021 Sep;200:38-47. doi: 10.1016/j.toxicon.2021.06.020. Epub 2021 Jul 6. Toxicon. 2021. PMID: 34237340
-
Sticholysins, pore-forming proteins from a marine anemone can induce maturation of dendritic cells through a TLR4 dependent-pathway.Mol Immunol. 2021 Mar;131:144-154. doi: 10.1016/j.molimm.2020.12.032. Epub 2021 Jan 6. Mol Immunol. 2021. PMID: 33422341
-
Decoupling immunomodulatory properties from lipid binding in the α-pore-forming toxin Sticholysin II.Int J Biol Macromol. 2024 Nov;280(Pt 4):136244. doi: 10.1016/j.ijbiomac.2024.136244. Epub 2024 Oct 3. Int J Biol Macromol. 2024. PMID: 39368578
-
Sticholysins, two pore-forming toxins produced by the Caribbean Sea anemone Stichodactyla helianthus: their interaction with membranes.Toxicon. 2009 Dec 15;54(8):1135-47. doi: 10.1016/j.toxicon.2009.02.022. Epub 2009 Mar 4. Toxicon. 2009. PMID: 19268489 Review.
Cited by
-
Actinoporin-like Proteins Are Widely Distributed in the Phylum Porifera.Mar Drugs. 2022 Jan 15;20(1):74. doi: 10.3390/md20010074. Mar Drugs. 2022. PMID: 35049929 Free PMC article.
-
Actinoporins: From the Structure and Function to the Generation of Biotechnological and Therapeutic Tools.Biomolecules. 2020 Apr 2;10(4):539. doi: 10.3390/biom10040539. Biomolecules. 2020. PMID: 32252469 Free PMC article. Review.
-
Membrane Remodeling by the Lytic Fragment of SticholysinII: Implications for the Toroidal Pore Model.Biophys J. 2019 Nov 5;117(9):1563-1576. doi: 10.1016/j.bpj.2019.09.018. Epub 2019 Sep 20. Biophys J. 2019. PMID: 31587828 Free PMC article.
-
The Important Role of Membrane Fluidity on the Lytic Mechanism of the α-Pore-Forming Toxin Sticholysin I.Toxins (Basel). 2023 Jan 16;15(1):80. doi: 10.3390/toxins15010080. Toxins (Basel). 2023. PMID: 36668899 Free PMC article.
-
Panorama of the Intracellular Molecular Concert Orchestrated by Actinoporins, Pore-Forming Toxins from Sea Anemones.Toxins (Basel). 2021 Aug 13;13(8):567. doi: 10.3390/toxins13080567. Toxins (Basel). 2021. PMID: 34437438 Free PMC article. Review.
References
-
- Kaliński P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 1998;161:2804–2809. - PubMed
-
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. - PubMed
-
- Kurts C, Robinson BWS, Knolle PA. Cross-priming in health and disease. Nat. Rev. Immunol. 2010;10:403–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

