Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants
- PMID: 28258204
- PMCID: PMC5379917
- DOI: 10.1085/jgp.201611668
Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants
Abstract
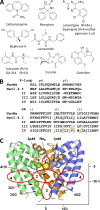
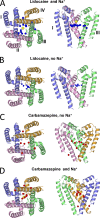
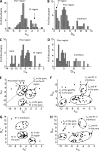
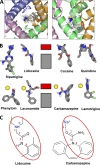
Local anesthetics, antiarrhythmics, and anticonvulsants include both charged and electroneutral compounds that block voltage-gated sodium channels. Prior studies have revealed a common drug-binding region within the pore, but details about the binding sites and mechanism of block remain unclear. Here, we use the x-ray structure of a prokaryotic sodium channel, NavMs, to model a eukaryotic channel and dock representative ligands. These include lidocaine, QX-314, cocaine, quinidine, lamotrigine, carbamazepine (CMZ), phenytoin, lacosamide, sipatrigine, and bisphenol A. Preliminary calculations demonstrated that a sodium ion near the selectivity filter attracts electroneutral CMZ but repels cationic lidocaine. Therefore, we further docked electroneutral and cationic drugs with and without a sodium ion, respectively. In our models, all the drugs interact with a phenylalanine in helix IVS6. Electroneutral drugs trap a sodium ion in the proximity of the selectivity filter, and this same site attracts the charged group of cationic ligands. At this position, even small drugs can block the permeation pathway by an electrostatic or steric mechanism. Our study proposes a common pharmacophore for these diverse drugs. It includes a cationic moiety and an aromatic moiety, which are usually linked by four bonds.
© 2017 Tikhonov and Zhorov.
Figures



Comment in
-
Sodium ion unlocks understanding of channel blockade.J Gen Physiol. 2017 Apr 3;149(4):405. doi: 10.1085/jgp.201711783. Epub 2017 Mar 13. J Gen Physiol. 2017. PMID: 28289068 Free PMC article.
References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

