Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis
- PMID: 28258218
- PMCID: PMC5399120
- DOI: 10.1074/jbc.M117.778308
Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis
Abstract
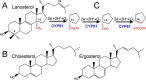
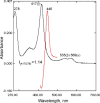
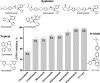
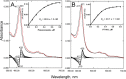
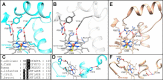
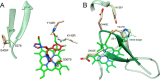
With some advances in modern medicine (such as cancer chemotherapy, broad exposure to antibiotics, and immunosuppression), the incidence of opportunistic fungal pathogens such as Candida albicans has increased. Cases of drug resistance among these pathogens have become more frequent, requiring the development of new drugs and a better understanding of the targeted enzymes. Sterol 14α-demethylase (CYP51) is a cytochrome P450 enzyme required for biosynthesis of sterols in eukaryotic cells and is the major target of clinical drugs for managing fungal pathogens, but some of the CYP51 key features important for rational drug design have remained obscure. We report the catalytic properties, ligand-binding profiles, and inhibition of enzymatic activity of C. albicans CYP51 by clinical antifungal drugs that are used systemically (fluconazole, voriconazole, ketoconazole, itraconazole, and posaconazole) and topically (miconazole and clotrimazole) and by a tetrazole-based drug candidate, VT-1161 (oteseconazole: (R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1-(5-(4-(2,2,2-trifluoroethoxy)phenyl)pyridin-2-yl)propan-2-ol). Among the compounds tested, the first-line drug fluconazole was the weakest inhibitor, whereas posaconazole and VT-1161 were the strongest CYP51 inhibitors. We determined the X-ray structures of C. albicans CYP51 complexes with posaconazole and VT-1161, providing a molecular mechanism for the potencies of these drugs, including the activity of VT-1161 against Candida krusei and Candida glabrata, pathogens that are intrinsically resistant to fluconazole. Our comparative structural analysis outlines phylum-specific CYP51 features that could direct future rational development of more efficient broad-spectrum antifungals.
Keywords: Candida albicans; X-ray crystallography; antifungal drugs; cytochrome P450; enzyme inhibitor; enzyme kinetics; sterol 14 alpha-demethylase (CYP51); sterol biosynthesis.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures








References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

