CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells
- PMID: 28258248
- PMCID: PMC5469328
- DOI: 10.1042/BSR20160470
CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells
Abstract
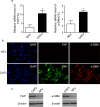
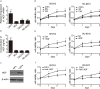
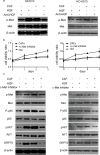
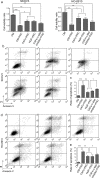
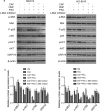
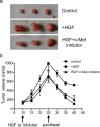
The tumour microenvironment is a highly heterogeneous entity that plays crucial roles in cancer progression. As the most prominent stromal cell types, cancer-associated fibroblasts (CAFs) produce a variety of factors into the tumour microenvironment. In the present study, we firstly isolated CAFs from tumour tissues of the patients with ovarian cancer and demonstrated that the hepatocyte growth factor (HGF) was highly expressed in the supernatants of CAFs. CAF-derived HGF or human recombinant HGF promoted cell proliferation in human ovarian cell lines SKOV3 and HO-8910 cells. Western blotting analysis also showed that CAF-derived HGF or recombinant HGF activated c-Met/phosphoinositide 3-kinase (PI3K)/Akt and glucose-regulated protein 78 (GRP78) signalling pathways in ovarian cancer cells, and these effects could be abrogated by anti-HGF and c-Met inhibitor INCB28060. Moreover, HGF in CAF matrix attenuated paclitaxel (PAC)-caused inhibition of cell proliferation and increase in cell apoptosis through activating c-Met/PI3K/Akt and GRP78 pathways in SKOV3 and HO-8910 cells. The results in vitro were further validated in nude mice. These findings suggest that CAF-derived HGF plays crucial roles in cell proliferation and drug resistance in ovarian cancer cells.
Keywords: cancer-associated fibroblasts; cell proliferation; drug resistance; hepatocyte growth factor; ovarian cancer.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Cancer Associated Fibroblast-Derived Hepatocyte Growth Factor Inhibits the Paclitaxel-Induced Apoptosis of Lung Cancer A549 Cells by Up-Regulating the PI3K/Akt and GRP78 Signaling on a Microfluidic Platform.PLoS One. 2015 Jun 26;10(6):e0129593. doi: 10.1371/journal.pone.0129593. eCollection 2015. PLoS One. 2015. PMID: 26115510 Free PMC article.
-
Norcantharidin combined with EGFR-TKIs overcomes HGF-induced resistance to EGFR-TKIs in EGFR mutant lung cancer cells via inhibition of Met/PI3k/Akt pathway.Cancer Chemother Pharmacol. 2015 Aug;76(2):307-15. doi: 10.1007/s00280-015-2792-x. Epub 2015 Jun 11. Cancer Chemother Pharmacol. 2015. PMID: 26063323
-
Transient PI3K inhibition induces apoptosis and overcomes HGF-mediated resistance to EGFR-TKIs in EGFR mutant lung cancer.Clin Cancer Res. 2011 Apr 15;17(8):2260-9. doi: 10.1158/1078-0432.CCR-10-1993. Epub 2011 Jan 10. Clin Cancer Res. 2011. PMID: 21220474
-
HGF/MET signaling in ovarian cancer.Curr Mol Med. 2008 Sep;8(6):469-80. doi: 10.2174/156652408785747933. Curr Mol Med. 2008. PMID: 18781954 Review.
-
Regulation of HGF and c-MET Interaction in Normal Ovary and Ovarian Cancer.Reprod Sci. 2017 Apr;24(4):494-501. doi: 10.1177/1933719116648212. Epub 2016 Sep 27. Reprod Sci. 2017. PMID: 27170665 Free PMC article. Review.
Cited by
-
The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment.Front Cell Dev Biol. 2021 May 20;9:637675. doi: 10.3389/fcell.2021.637675. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095111 Free PMC article. Review.
-
The Perfect Combination: Enhancing Patient Response to PD-1-Based Therapies in Epithelial Ovarian Cancer.Cancers (Basel). 2020 Aug 3;12(8):2150. doi: 10.3390/cancers12082150. Cancers (Basel). 2020. PMID: 32756436 Free PMC article. Review.
-
Cancer-Associated Fibroblasts Promote the Upregulation of PD-L1 Expression Through Akt Phosphorylation in Colorectal Cancer.Front Oncol. 2021 Nov 19;11:748465. doi: 10.3389/fonc.2021.748465. eCollection 2021. Front Oncol. 2021. PMID: 34868949 Free PMC article.
-
Cancer-associated fibroblast-secreted FGF7 as an ovarian cancer progression promoter.J Transl Med. 2024 Mar 15;22(1):280. doi: 10.1186/s12967-024-05085-y. J Transl Med. 2024. PMID: 38491511 Free PMC article.
-
Cancer associated fibroblasts serve as an ovarian cancer stem cell niche through noncanonical Wnt5a signaling.NPJ Precis Oncol. 2024 Jan 8;8(1):7. doi: 10.1038/s41698-023-00495-5. NPJ Precis Oncol. 2024. PMID: 38191909 Free PMC article.
References
-
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–86 - PubMed
-
- Siegel R.L., Miller K.D. and Jemal A. (2015) Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29 - PubMed
-
- Vergote I., Trope C.G., Amant F., Kristensen G.B., Ehlen T., Johnson N. et al. (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 363, 943–953 - PubMed
-
- Orimo A. and Weinberg R.A. (2006) Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5, 1597–1601 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

