In Vitro Diagnosis of Immediate Drug Hypersensitivity Anno 2017: Potentials and Limitations
- PMID: 28258478
- PMCID: PMC5427047
- DOI: 10.1007/s40268-017-0176-x
In Vitro Diagnosis of Immediate Drug Hypersensitivity Anno 2017: Potentials and Limitations
Abstract
Background: For most physicians, quantification of drug-specific immunoglobulin E (drug-sIgE) antibodies constitutes the primary in vitro measure to document immediate drug hypersensitivity reactions (IDHR). Unfortunately, this is often insufficient to correctly identify patients with IgE-mediated IDHR and impossible for non-IgE-mediated IDHR that result from alternative routes of basophil and mast cell activation. In these difficult cases, diagnosis might benefit from cellular tests such as basophil activation tests (BAT).
Aim: The aim was to review the potential and limitations of quantification of sIgE and BAT in diagnosing IDHR. The utility of quantification of serum tryptase is discussed.
Methods: A literature search was conducted using the key words allergy, basophil activation, CD63, CD203c, diagnosis, drugs, hypersensitivity, flow cytometry, specific IgE antibodies; this was complemented by the authors' own experience.
Results: The drugs that have been most studied with both techniques are β-lactam antibiotics and curarizing neuromuscular blocking agents (NMBA). For sIgE morphine, data are available on the value of this test as a biomarker for sensitization to substituted ammonium structures that constitute the major epitope of NMBA, especially rocuronium and suxamethonium. For the BAT, there are also data on non-steroidal anti-inflammatory drugs (NSAIDs) and iodinated radiocontrast media. For β-lactam antibiotics, sensitivity and specificity of sIgE varies between 0 and 85% and 52 and 100%, respectively. For NMBA, sensitivity and specificity varies between 38.5 and 92% and 85.7 and 100%, respectively. Specific IgE to morphine should not be used in isolation to diagnose IDHR to NMBA nor opiates. For the BAT, sensitivity generally varies between 50 and 60%, whereas specificity attains 80%, except for quinolones and NSAIDs.
Conclusions: Although drug-sIgE assays and BAT can provide useful information in the diagnosis of IDHR, their predictive value is not absolute. Large-scale collaborative studies are mandatory to harmonize and optimize test protocols and to establish drug-specific decision thresholds.
Conflict of interest statement
IID, EAM, ALVG, KC, AU, MF, VS, CHB, CM, MMH, LSDC and DGE declare they have no conflict of interest. No funding for this review was provided.
Figures
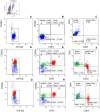
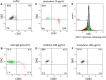
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

