Tim-3 and its role in regulating anti-tumor immunity
- PMID: 28258697
- PMCID: PMC5512889
- DOI: 10.1111/imr.12520
Tim-3 and its role in regulating anti-tumor immunity
Abstract
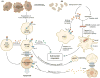
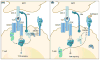
Immunotherapy is being increasingly recognized as a key therapeutic modality to treat cancer and represents one of the most exciting treatments for the disease. Fighting cancer with immunotherapy has revolutionized treatment for some patients and therapies targeting the immune checkpoint molecules such as CTLA-4 and PD-1 have achieved durable responses in melanoma, renal cancer, Hodgkin's diseases and lung cancer. However, the success rate of these treatments has been low and a large number of cancers, including colorectal cancer remain largely refractory to CTLA-4 and PD-1 blockade. This has provided impetus to identify other co-inhibitory receptors that could be exploited to enhance response rates of current immunotherapeutic agents and achieve responses to the cancers that are refectory to immunotherapy. Tim-3 is a co-inhibitory receptor that is expressed on IFN-g-producing T cells, FoxP3+ Treg cells and innate immune cells (macrophages and dendritic cells) where it has been shown to suppress their responses upon interaction with their ligand(s). Tim-3 has gained prominence as a potential candidate for cancer immunotherapy, where it has been shown that in vivo blockade of Tim-3 with other check-point inhibitors enhances anti-tumor immunity and suppresses tumor growth in several preclinical tumor models. This review discusses the recent findings on Tim-3, the role it plays in regulating immune responses in different cell types and the rationale for targeting Tim-3 for effective cancer immunotherapy.
Keywords: T cell exhaustion; Tim-3; checkpoint blockade; checkpoint inhibitors; immunotherapy; tumor immunity.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests.
Figures


References
-
- Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. - PubMed
-
- Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11:362–369. - PubMed
-
- McIntire JJ, Umetsu SE, Akbari O, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

