Association between Use of Prophylactic Indomethacin and the Risk for Bronchopulmonary Dysplasia in Extremely Preterm Infants
- PMID: 28258737
- PMCID: PMC5484725
- DOI: 10.1016/j.jpeds.2017.02.003
Association between Use of Prophylactic Indomethacin and the Risk for Bronchopulmonary Dysplasia in Extremely Preterm Infants
Abstract
Objective: To assess the association between prophylactic indomethacin and bronchopulmonary dysplasia (BPD) in a recent, large cohort of extremely preterm infants.
Study design: Retrospective cohort study using prospectively collected data for infants with gestational ages < 29 weeks or birth weights of 401-1000 g born between 2008 and 2012 at participating hospitals of the National Institute of Child Health and Human Development Neonatal Research Network. Infants treated with indomethacin in the first 24 hours of life were compared with those who were not. Study outcomes were BPD, defined as use of supplemental oxygen at 36 weeks postmenstrual age among survivors to that time point, death, and the composite of death or BPD. Prespecified subgroup analyses were performed.
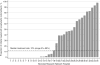
Results: Prophylactic indomethacin use varied by hospital. Treatment of a patent ductus arteriosus after the first day of life was less common among 2587 infants who received prophylactic indomethacin compared with 5244 who did not (21.0% vs 36.1%, P < .001). After adjustment for potential confounders, use of prophylactic indomethacin was not associated with higher or lower odds of BPD (OR 0.89, 95% CI 0.72-1.10), death (OR 0.80, 95% CI 0.64-1.01), or death or BPD (OR 0.87, 95% CI 0.71-1.05). The only evidence of subgroup effects associated with prophylactic indomethacin were lower odds of death among infants with birth weights above the 10th percentile and those who were not treated for a patent ductus arteriosus after the first day of life.
Conclusions: Prophylactic indomethacin was not associated with either reduced or increased risk for BPD or death.
Trial registration: ClinicalTrials.gov: NCT00063063.
Keywords: bronchopulmonary dysplasia; extreme prematurity; indomethacin; prophylaxis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Comment in
-
Prophylactic Indomethacin Revisited.J Pediatr. 2017 Jul;186:11-14.e1. doi: 10.1016/j.jpeds.2017.03.036. Epub 2017 Apr 7. J Pediatr. 2017. PMID: 28396028 Free PMC article.
References
-
- Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: Time to accept the null hypothesis? J Perinatol. 2010;30:241–52. - PubMed
-
- Benitz WE. Patent ductus arteriosus: To treat or not to treat? Arch Dis Child Fetal Neonatal Ed. 2012;97:F80–F2. - PubMed
-
- Palta M, Gabbert D, Weinstein MR, Peters ME. Multivariate assessment of traditional risk factors for chronic lung disease in very low birth weight neonates. The Newborn Lung Project. J Pediatr. 1991;119:285–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 HD068263/HD/NICHD NIH HHS/United States
- U10 HD027856/HD/NICHD NIH HHS/United States
- UG1 HD087226/HD/NICHD NIH HHS/United States
- U10 HD021364/HD/NICHD NIH HHS/United States
- UG1 HD027853/HD/NICHD NIH HHS/United States
- UG1 HD087229/HD/NICHD NIH HHS/United States
- U10 HD040492/HD/NICHD NIH HHS/United States
- U10 HD027904/HD/NICHD NIH HHS/United States
- U10 HD068244/HD/NICHD NIH HHS/United States
- UG1 HD027904/HD/NICHD NIH HHS/United States
- UG1 HD040492/HD/NICHD NIH HHS/United States
- UG1 HD021364/HD/NICHD NIH HHS/United States
- UG1 HD027880/HD/NICHD NIH HHS/United States
- U10 HD068284/HD/NICHD NIH HHS/United States
- U10 HD068278/HD/NICHD NIH HHS/United States
- UG1 HD027851/HD/NICHD NIH HHS/United States
- UG1 HD068278/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

