Conservation of estrogen receptor function in invertebrate reproduction
- PMID: 28259146
- PMCID: PMC5336670
- DOI: 10.1186/s12862-017-0909-z
Conservation of estrogen receptor function in invertebrate reproduction
Abstract
Background: Rotifers are microscopic aquatic invertebrates that reproduce both sexually and asexually. Though rotifers are phylogenetically distant from humans, and have specialized reproductive physiology, this work identifies a surprising conservation in the control of reproduction between humans and rotifers through the estrogen receptor. Until recently, steroid signaling has been observed in only a few invertebrate taxa and its role in regulating invertebrate reproduction has not been clearly demonstrated. Insights into the evolution of sex signaling pathways can be gained by clarifying how receptors function in invertebrate reproduction.
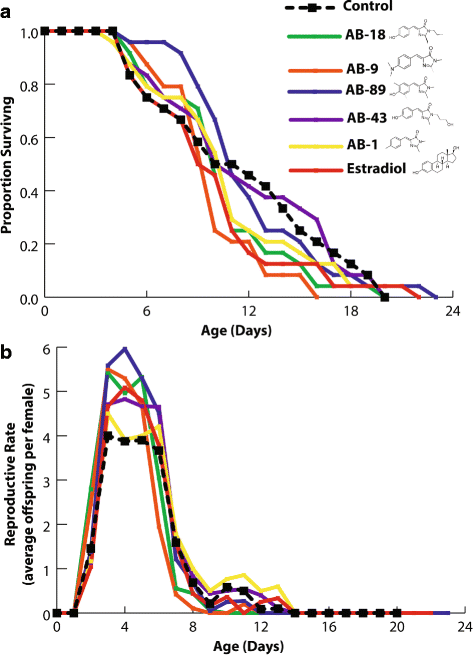
Results: In this paper, we show that a ligand-activated estrogen-like receptor in rotifers binds human estradiol and regulates reproductive output in females. In other invertebrates characterized thus far, ER ligand binding domains have occluded ligand-binding sites and the ERs are not ligand activated. We have used a suite of computational, biochemical and biological techniques to determine that the rotifer ER binding site is not occluded and can bind human estradiol.
Conclusions: Our results demonstrate that this mammalian hormone receptor plays a key role in reproduction of the ancient microinvertebrate Brachinous manjavacas. The presence and activity of the ER within the phylum Rotifera indicates that the ER structure and function is highly conserved throughout animal evolution.
Keywords: Estrogen receptor; Receptor; Rotifera.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

