Architecture of the Flagellar Switch Complex of Escherichia coli: Conformational Plasticity of FliG and Implications for Adaptive Remodeling
- PMID: 28259628
- PMCID: PMC5494207
- DOI: 10.1016/j.jmb.2017.02.014
Architecture of the Flagellar Switch Complex of Escherichia coli: Conformational Plasticity of FliG and Implications for Adaptive Remodeling
Abstract
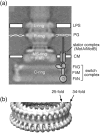
Structural models of the complex that regulates the direction of flagellar rotation assume either ~34 or ~25 copies of the protein FliG. Support for ~34 came from crosslinking experiments identifying an intersubunit contact most consistent with that number; support for ~25 came from the observation that flagella can assemble and rotate when FliG is genetically fused to FliF, for which the accepted number is ~25. Here, we have undertaken crosslinking and other experiments to address more fully the question of FliG number. The results indicate a copy number of ~25 for FliG. An interaction between the C-terminal and middle domains, which has been taken to support a model with ~34 copies, is also supported. To reconcile the interaction with a FliG number of ~25, we hypothesize conformational plasticity in an interdomain segment of FliG that allows some subunits to bridge gaps created by the number mismatch. This proposal is supported by mutant phenotypes and other results indicating that the normally helical segment adopts a more extended conformation in some subunits. The FliG amino-terminal domain is organized in a regular array with dimensions matching a ring in the upper part of the complex. The model predicts that FliG copy number should be tied to that of FliF, whereas FliM copy number can increase or decrease according to the number of FliG subunits that adopt the extended conformation. This has implications for the phenomenon of adaptive switch remodeling, in which the FliM copy number varies to adjust the bias of the switch.
Keywords: chemotaxis; molecular motors; motility; protein structure; self-assembly.
Copyright © 2017. Published by Elsevier Ltd.
Figures







References
-
- Berg HC, Anderson RA. Bacteria swim by rotating their flagellar filaments. Nature. 1973;245:380–382. - PubMed
-
- Hirota N, Kitada M, Imae Y. Flagellar motors of alkalophilic Bacillus are powered by an electrochemical potential gradient of Na+ FEBS Lett. 1981;132:278–280.
-
- Larsen SH, Reader RW, Kort EN, Tso WW, Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in E. coli. Nature. 1974;249:74–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

