Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice
- PMID: 28259806
- PMCID: PMC5639911
- DOI: 10.1016/j.physbeh.2017.02.044
Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice
Abstract
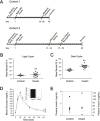
Intranasal insulin delivery is currently being used in clinical trials to test for improvement in human memory and cognition, and in particular, for lessening memory loss attributed to neurodegenerative diseases. Studies have reported the effects of short-term intranasal insulin treatment on various behaviors, but less have examined long-term effects. The olfactory bulb contains the highest density of insulin receptors in conjunction with the highest level of insulin transport within the brain. Previous research from our laboratory has demonstrated that acute insulin intranasal delivery (IND) enhanced both short- and long-term memory as well as increased two-odor discrimination in a two-choice paradigm. Herein, we investigated the behavioral and physiological effects of chronic insulin IND. Adult, male C57BL6/J mice were intranasally treated with 5μg/μl of insulin twice daily for 30 and 60days. Metabolic assessment indicated no change in body weight, caloric intake, or energy expenditure following chronic insulin IND, but an increase in the frequency of meal bouts selectively in the dark cycle. Unlike acute insulin IND, which has been shown to cause enhanced performance in odor habituation/dishabituation and two-odor discrimination tasks in mice, chronic insulin IND did not enhance olfactometry-based odorant discrimination or olfactory reversal learning. In an object memory recognition task, insulin IND-treated mice did not perform differently than controls, regardless of task duration. Biochemical analyses of the olfactory bulb revealed a modest 1.3 fold increase in IR kinase phosphorylation but no significant increase in Kv1.3 phosphorylation. Substrate phosphorylation of IR kinase downstream effectors (MAPK/ERK and Akt signaling) proved to be highly variable. These data indicate that chronic administration of insulin IND in mice fails to enhance olfactory ability, object memory recognition, or a majority of systems physiology metabolic factors - as reported to elicit a modulatory effect with acute administration. This leads to two alternative interpretations regarding long-term insulin IND in mice: 1) It causes an initial stage of insulin resistance to dampen the behaviors that would normally be modulated under acute insulin IND, but ability to clear a glucose challenge is still retained, or 2) There is a lack of behavioral modulation at high concentration of insulin attributed to the twice daily intervals of hyperinsulinemia caused by insulin IND administration without any insulin resistance, per se.
Keywords: IR kinase; Intranasal; Kv1.3; Meal frequency; Olfactometry; Olfactory.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

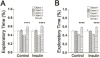

References
-
- Marks DR. Kv13 Modulation by PSD-96 and Insulin. Florida State University; 2008.
-
- Heni M, Kullmann S, Preissl H, Fritsche A, Haring HU. Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol. 2015;11:701–711. - PubMed
-
- Cardoso S, Correia S, Santos RX, Carvalho C, Santos MS, Oliveira CR, Perry G, Smith MA, Zhu X, Moreira PI. Insulin is a two-edged knife on the brain. J Alzheimers Dis. 2009;18:483–507. - PubMed
-
- Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44:897–906. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

