Ethyl acetate extract from Asparagus cochinchinensis exerts anti‑inflammatory effects in LPS‑stimulated RAW264.7 macrophage cells by regulating COX‑2/iNOS, inflammatory cytokine expression, MAP kinase pathways, the cell cycle and anti-oxidant activity
- PMID: 28260011
- PMCID: PMC5364973
- DOI: 10.3892/mmr.2017.6166
Ethyl acetate extract from Asparagus cochinchinensis exerts anti‑inflammatory effects in LPS‑stimulated RAW264.7 macrophage cells by regulating COX‑2/iNOS, inflammatory cytokine expression, MAP kinase pathways, the cell cycle and anti-oxidant activity
Abstract
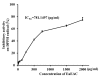
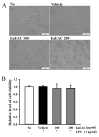
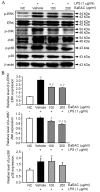
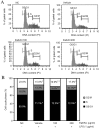
Asparagus cochinchinesis (A. cochinchinesis) is a medicine traditionally used to treat fever, cough, kidney disease, breast cancer, inflammatory disease and brain disease in northeast Asian countries. Although numerous studies of the anti‑inflammatory effects of A. cochinchinesis have been conducted, the underlying mechanisms of such effects in macrophages remain to be demonstrated. To investigate the mechanism of suppressive effects on the inflammatory response in macrophages, alterations of the nitric oxide (NO) level, the cell viability, inducible nitric oxide synthase (iNOS) and cyclooxygenase‑2 (COX‑2) expression levels, inflammatory cytokine expression, the mitogen-activated protein kinase (MAPK) signaling pathway, cell cycle arrest and reactive oxygen species (ROS) levels were measured in lipopolysaccharide (LPS)-activated RAW264.7 cells following treatment with ethyl acetate extract from A. cochinchinesis root (EaEAC). RAW264.7 cells pretreated two different concentrations of EaEAC prior to LPS treatment exhibited no significant toxicity. The concentration of NO was significantly decreased in the EaEAC + LPS treated group compared with the vehicle + LPS treated group. A similar decrease in mRNA transcript level of COX‑2, iNOS, pro-inflammatory cytokines [tumor necrosis factor‑α and interleukin (IL)‑1β] and anti‑inflammatory cytokines (IL‑6 and IL‑10) was detected in the EaEAC + LPS treated group compared with the vehicle + LPS treated group, although the decrease rate varied. Enhancement of the phosphorylation of MAPK family members following LPS treatment was partially rescued in the EaEAC pretreated group, and the cell cycle was arrested at the G2/M phase. Furthermore, the EaEAC pretreated group exhibited a reduced level of ROS generation compared with the vehicle + LPS treated group. Taken together, these results suggest that EaEAC suppresses inflammatory responses through inhibition of NO production, COX‑2 expression and ROS production, as well as differential regulation of inflammatory cytokines and cell cycle in RAW264.7 cells. In addition, these results provide strong evidence to suggest that EaEAC may be considered as an important candidate for the treatment of particular inflammatory diseases.
Figures


References
-
- Xiao PG. Modern Chinese material medica. Chemical Industry Press; Beijing: 2002. p. 150.
-
- Liu YZ, Qu FY, Zhang PX. Effect of chloroform extract of Tiandong on the brain antioxidation of D-galatose-induced senile mice. Heilongjiang Med Pharm. 2001;24:7–8.
-
- Ni JM, Zhao R, Wang R. Comparison on amino acid content in prepared and unprepared Asparagus cochinchinensis. Chin Tradit Herb Drugs. 1992;23:182–183.
-
- Tenji K, Junzo S. Studies on the constituents of Asparagi Radix. I. On the structures of furostanol oligosides of Asparagus cochinchinensis (LOUREIO) MERRILL. Chem Pharm Bull. 1979;27:3086–3094. doi: 10.1248/cpb.27.3086. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

