Silencing BMI1 radiosensitizes human breast cancer cells by inducing DNA damage and autophagy
- PMID: 28260023
- PMCID: PMC5367353
- DOI: 10.3892/or.2017.5478
Silencing BMI1 radiosensitizes human breast cancer cells by inducing DNA damage and autophagy
Abstract
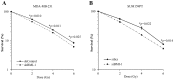
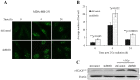
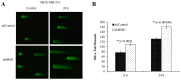
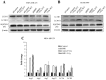
Overexpression of BMI1 in human cancer cells, a member of the polycomb group of repressive complexes, correlates with advanced stage of disease, aggressive clinico-pathological behavior, poor prognosis, and resistance to radiation and chemotherapy. Studies have shown that experimental reduction of BMI1 protein level in tumor cells results in inhibition of cell proliferation, induction of apoptosis and/or senescence, and increased susceptibility to cytotoxic agents and radiation therapy. Although a role for BMI1 in cancer progression and its importance as a molecular target for cancer therapy has been established, information on the impact of silencing BMI1 in triple-negative breast cancer (TNBC) and its consequence on radiotherapy have not been well studied. Therefore, in the present study we investigated the potential therapeutic benefit of radiation therapy in BMI1-silenced breast cancer cells and studied the mechanism(s) of radiosensitization. Human MDA-MB-231 and SUM159PT breast cancer cells that were either stably transfected with a lentiviral vector expressing BMI1 shRNA (shBMI1) or control shRNA (shControl) or transient transfection with a BMI1-specific siRNA were used. Silencing of BMI1 resulted in marked reduction in BMI1 both at the mRNA and protein level that was accompanied by a significant reduction in cell migration compared to control cells. Further, BMI1 knockdown produced a marked enhancement of DNA damage as evidenced by Comet Assay and γH2AX foci, resulting in a dose-dependent radiosensitization effect. Molecular studies revealed modulation of protein expression that is associated with the DNA damage response (DDR) and autophagy pathways. Our results demonstrate that BMI1 is an important therapeutic target in breast cancer and suppression of BMI1 produces radiation sensitivity. Further, combining BMI1-targeted therapeutics with radiation might benefit patients diagnosed with TNBC.
Figures


Similar articles
-
BMI1 and PTEN are key determinants of breast cancer therapy: A plausible therapeutic target in breast cancer.Gene. 2018 Dec 15;678:302-311. doi: 10.1016/j.gene.2018.08.022. Epub 2018 Aug 8. Gene. 2018. PMID: 30096458 Review.
-
HuR silencing elicits oxidative stress and DNA damage and sensitizes human triple-negative breast cancer cells to radiotherapy.Oncotarget. 2016 Oct 4;7(40):64820-64835. doi: 10.18632/oncotarget.11706. Oncotarget. 2016. PMID: 27588488 Free PMC article.
-
Regorafenib sensitizes human breast cancer cells to radiation by inhibiting multiple kinases and inducing DNA damage.Int J Radiat Biol. 2021;97(8):1109-1120. doi: 10.1080/09553002.2020.1730012. Epub 2020 Mar 2. Int J Radiat Biol. 2021. PMID: 32052681 Free PMC article.
-
Radiosensitization with combined use of olaparib and PI-103 in triple-negative breast cancer.BMC Cancer. 2015 Mar 3;15:89. doi: 10.1186/s12885-015-1090-7. BMC Cancer. 2015. PMID: 25884663 Free PMC article.
-
BMI1 in the heart: Novel functions beyond tumorigenesis.EBioMedicine. 2021 Jan;63:103193. doi: 10.1016/j.ebiom.2020.103193. Epub 2021 Jan 6. EBioMedicine. 2021. PMID: 33421944 Free PMC article. Review.
Cited by
-
Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies.Front Immunol. 2020 Aug 7;11:1280. doi: 10.3389/fimmu.2020.01280. eCollection 2020. Front Immunol. 2020. PMID: 32849491 Free PMC article. Review.
-
Total saponins of Bolbostemma paniculatum (maxim.) Franquet exert antitumor activity against MDA-MB-231 human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway.BMC Complement Altern Med. 2019 Nov 8;19(1):304. doi: 10.1186/s12906-019-2708-0. BMC Complement Altern Med. 2019. PMID: 31703679 Free PMC article.
-
The Crucial Roles of Bmi-1 in Cancer: Implications in Pathogenesis, Metastasis, Drug Resistance, and Targeted Therapies.Int J Mol Sci. 2022 Jul 26;23(15):8231. doi: 10.3390/ijms23158231. Int J Mol Sci. 2022. PMID: 35897796 Free PMC article. Review.
-
A novel BMI-1 inhibitor QW24 for the treatment of stem-like colorectal cancer.J Exp Clin Cancer Res. 2019 Oct 22;38(1):422. doi: 10.1186/s13046-019-1392-8. J Exp Clin Cancer Res. 2019. PMID: 31640758 Free PMC article.
-
Mechanisms of radioresistance and radiosensitization strategies for Triple Negative Breast Cancer.Transl Oncol. 2025 May;55:102351. doi: 10.1016/j.tranon.2025.102351. Epub 2025 Mar 19. Transl Oncol. 2025. PMID: 40112501 Free PMC article. Review.
References
-
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: Knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. - PubMed
-
- Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, Hulsman D, Leung C, Arsenijevic Y, Marino S, et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005;19:1438–1443. doi: 10.1101/gad.1299305. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

