The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response
- PMID: 28260504
- PMCID: PMC5930453
- DOI: 10.2174/1874467210666170302104053
The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response
Abstract
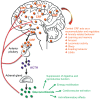
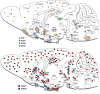
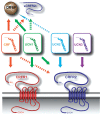
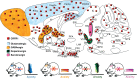
Background: Dysregulated stress neurocircuits, caused by genetic and/or environmental changes, underlie the development of many neuropsychiatric disorders. Corticotropin-releasing factor (CRF) is the major physiological activator of the hypothalamic-pituitary-adrenal (HPA) axis and consequently a primary regulator of the mammalian stress response. Together with its three family members, urocortins (UCNs) 1, 2, and 3, CRF integrates the neuroendocrine, autonomic, metabolic and behavioral responses to stress by activating its cognate receptors CRFR1 and CRFR2.
Objective: Here we review the past and current state of the CRF/CRFR field, ranging from pharmacological studies to genetic mouse models and virus-mediated manipulations.
Results: Although it is well established that CRF/CRFR1 signaling mediates aversive responses, including anxiety and depression-like behaviors, a number of recent studies have challenged this viewpoint by revealing anxiolytic and appetitive properties of specific CRF/CRFR1 circuits. In contrast, the UCN/CRFR2 system is less well understood and may possibly also exert divergent functions on physiology and behavior depending on the brain region, underlying circuit, and/or experienced stress conditions.
Conclusion: A plethora of available genetic tools, including conventional and conditional mouse mutants targeting CRF system components, has greatly advanced our understanding about the endogenous mechanisms underlying HPA system regulation and CRF/UCN-related neuronal circuits involved in stress-related behaviors. Yet, the detailed pathways and molecular mechanisms by which the CRF/UCN-system translates negative or positive stimuli into the final, integrated biological response are not completely understood. The utilization of future complementary methodologies, such as cell-type specific Cre-driver lines, viral and optogenetic tools will help to further dissect the function of genetically defined CRF/UCN neurocircuits in the context of adaptive and maladaptive stress responses.
Keywords: Corticotropin-releasing factor; hypothalamic-pituitary-adrenal (HPA); mouse genetic tools; neuropsychiatric disorders; stress; urocortin.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures
Similar articles
-
Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior.J Neurosci. 2002 Jan 1;22(1):193-9. doi: 10.1523/JNEUROSCI.22-01-00193.2002. J Neurosci. 2002. PMID: 11756502 Free PMC article.
-
The CRF/Urocortin systems as therapeutic targets for alcohol use disorders.Int Rev Neurobiol. 2024;178:97-152. doi: 10.1016/bs.irn.2024.08.002. Epub 2024 Aug 28. Int Rev Neurobiol. 2024. PMID: 39523064 Review.
-
Urocortins: CRF's siblings and their potential role in anxiety, depression and alcohol drinking behavior.Alcohol. 2012 Jun;46(4):349-57. doi: 10.1016/j.alcohol.2011.10.007. Epub 2012 Mar 22. Alcohol. 2012. PMID: 22444954 Free PMC article. Review.
-
Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids.Mol Endocrinol. 2005 Feb;19(2):441-58. doi: 10.1210/me.2004-0300. Epub 2004 Oct 28. Mol Endocrinol. 2005. PMID: 15514029
-
Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors.Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2843-8. doi: 10.1073/pnas.051626398. Proc Natl Acad Sci U S A. 2001. PMID: 11226328 Free PMC article.
Cited by
-
Role of canonical and non-canonical cAMP sources in CRHR2α-dependent signaling.PLoS One. 2024 Oct 2;19(10):e0310699. doi: 10.1371/journal.pone.0310699. eCollection 2024. PLoS One. 2024. PMID: 39356686 Free PMC article.
-
A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism.Int J Mol Sci. 2019 Sep 5;20(18):4348. doi: 10.3390/ijms20184348. Int J Mol Sci. 2019. PMID: 31491880 Free PMC article.
-
Effects of neuromodulation on cognitive and emotional responses to psychosocial stressors in healthy humans.Neurobiol Stress. 2023 Jan 11;22:100515. doi: 10.1016/j.ynstr.2023.100515. eCollection 2023 Jan. Neurobiol Stress. 2023. PMID: 36691646 Free PMC article. Review.
-
How Metabolic State May Regulate Fear: Presence of Metabolic Receptors in the Fear Circuitry.Front Neurosci. 2018 Aug 27;12:594. doi: 10.3389/fnins.2018.00594. eCollection 2018. Front Neurosci. 2018. PMID: 30210279 Free PMC article.
-
Perturbations of Neuron-Restrictive Silencing Factor Modulate Corticotropin-Releasing Hormone Gene Expression in the Human Cell Line BeWo.Mol Neuropsychiatry. 2018 Oct;4(2):100-110. doi: 10.1159/000492635. Epub 2018 Sep 19. Mol Neuropsychiatry. 2018. PMID: 30397598 Free PMC article.
References
-
- Cohen S., Janicki-Deverts D., Miller G.E. Psychological stress and disease. JAMA. 2007;298:1685–1687. - PubMed
-
- Chrousos G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. - PubMed
-
- Adler N.E., Ostrove J.M. Socioeconomic status and health: what we know and what we don’t. Ann. N. Y. Acad. Sci. 1999;896:3–15. - PubMed
-
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

