Molecular design and synthesis of self-assembling camptothecin drug amphiphiles
- PMID: 28260797
- PMCID: PMC5520181
- DOI: 10.1038/aps.2016.151
Molecular design and synthesis of self-assembling camptothecin drug amphiphiles
Abstract
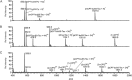
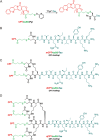
The conjugation of small molecular hydrophobic anticancer drugs onto a short peptide with overall hydrophilicity to create self-assembling drug amphiphiles offers a new prodrug strategy, producing well-defined, discrete nanostructures with a high and quantitative drug loading. Here we show the detailed synthesis procedure and how the molecular structure can influence the synthesis of the self-assembling prodrugs and the physicochemical properties of their assemblies. A series of camptothecin-based drug amphiphiles were synthesized via combined solid- and solution-phase synthetic techniques, and the physicochemical properties of their self-assembled nanostructures were probed using a number of imaging and spectroscopic techniques. We found that the number of incorporated drug molecules strongly influences the rate at which the drug amphiphiles are formed, exerting a steric hindrance toward any additional drugs to be conjugated and necessitating extended reaction time. The choice of peptide sequence was found to affect the solubility of the conjugates and, by extension, the critical aggregation concentration and contour length of the filamentous nanostructures formed. In the design of self-assembling drug amphiphiles, the number of conjugated drug molecules and the choice of peptide sequence have significant effects on the nanostructures formed. These observations may allow the fine-tuning of the physicochemical properties for specific drug delivery applications, ie systemic vs local delivery.
Figures




References
-
- Lien S, Lowman HB. Therapeutic peptides. Trends Biotechnol 2003; 21: 556–62. - PubMed
-
- Albericio F, Kruger HG. Therapeutic peptides. Future Med Chem 2012; 4: 1527–31. - PubMed
-
- Ahrens VM, Bellmann-Sickert K, Beck-Sickinger AG. Peptides and peptide conjugates: therapeutics on the upward path. Future Med Chem 2012; 4: 1567–86. - PubMed
-
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998; 279: 377–80. - PubMed
-
- Burkhart DJ, Kalet BT, Coleman MP, Post GC, Koch TH. Doxorubicin-formaldehyde conjugates targeting αvβ3 integrin. Mol Cancer Ther 2004; 3: 1593–604. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

