Molecular Mechanisms Involved in Schwann Cell Plasticity
- PMID: 28261057
- PMCID: PMC5314106
- DOI: 10.3389/fnmol.2017.00038
Molecular Mechanisms Involved in Schwann Cell Plasticity
Abstract
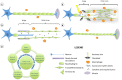
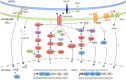
Schwann cell incredible plasticity is a hallmark of the utmost importance following nerve damage or in demyelinating neuropathies. After injury, Schwann cells undergo dedifferentiation before redifferentiating to promote nerve regeneration and complete functional recovery. This review updates and discusses the molecular mechanisms involved in the negative regulation of myelination as well as in the reprogramming of Schwann cells taking place early following nerve lesion to support repair. Significant advance has been made on signaling pathways and molecular components that regulate SC regenerative properties. These include for instance transcriptional regulators such as c-Jun or Notch, the MAPK and the Nrg1/ErbB2/3 pathways. This comprehensive overview ends with some therapeutical applications targeting factors that control Schwann cell plasticity and highlights the need to carefully modulate and balance this capacity to drive nerve repair.
Keywords: Schwann cell; molecular mechanisms; nerve injury; peripheral neuropathy; plasticity.
Figures
Similar articles
-
Transcriptional inhibition in Schwann cell development and nerve regeneration.Neural Regen Res. 2017 Aug;12(8):1241-1246. doi: 10.4103/1673-5374.213537. Neural Regen Res. 2017. PMID: 28966633 Free PMC article. Review.
-
Age-Dependent Schwann Cell Phenotype Regulation Following Peripheral Nerve Injury.J Hand Surg Asian Pac Vol. 2017 Dec;22(4):464-471. doi: 10.1142/S0218810417500514. J Hand Surg Asian Pac Vol. 2017. PMID: 29117831
-
Neural Progenitor-Like Cells Induced from Human Gingiva-Derived Mesenchymal Stem Cells Regulate Myelination of Schwann Cells in Rat Sciatic Nerve Regeneration.Stem Cells Transl Med. 2017 Feb;6(2):458-470. doi: 10.5966/sctm.2016-0177. Epub 2016 Sep 7. Stem Cells Transl Med. 2017. PMID: 28191764 Free PMC article.
-
Schwann cell O-GlcNAcylation promotes peripheral nerve remyelination via attenuation of the AP-1 transcription factor JUN.Proc Natl Acad Sci U S A. 2018 Jul 31;115(31):8019-8024. doi: 10.1073/pnas.1805538115. Epub 2018 Jul 16. Proc Natl Acad Sci U S A. 2018. PMID: 30012597 Free PMC article.
-
The Role of c-Jun and Autocrine Signaling Loops in the Control of Repair Schwann Cells and Regeneration.Front Cell Neurosci. 2022 Feb 9;15:820216. doi: 10.3389/fncel.2021.820216. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35221918 Free PMC article. Review.
Cited by
-
Integrated analysis of long noncoding RNAs and mRNA expression profiles reveals the potential role of lncRNAs in early stage of post-peripheral nerve injury in Sprague-Dawley rats.Aging (Albany NY). 2021 May 8;13(10):13909-13925. doi: 10.18632/aging.202989. Epub 2021 May 8. Aging (Albany NY). 2021. PMID: 33971626 Free PMC article.
-
Role of Connexin-Based Gap Junction Channels in Communication of Myelin Sheath in Schwann Cells.Front Cell Neurosci. 2019 Mar 1;13:69. doi: 10.3389/fncel.2019.00069. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30881289 Free PMC article. Review.
-
Effects of two intensities of treadmill exercise on neuromuscular recovery after median nerve crush injury in Wistar rats.J Exerc Rehabil. 2019 Jun 30;15(3):392-400. doi: 10.12965/jer.19.328126.063. eCollection 2019 Jun. J Exerc Rehabil. 2019. PMID: 31316931 Free PMC article.
-
Changes in the Coding and Non-coding Transcriptome and DNA Methylome that Define the Schwann Cell Repair Phenotype after Nerve Injury.Cell Rep. 2017 Sep 12;20(11):2719-2734. doi: 10.1016/j.celrep.2017.08.064. Cell Rep. 2017. PMID: 28903050 Free PMC article.
-
The Application of Stem Cells and Exosomes in Promoting Nerve Conduits for Peripheral Nerve Repair.Biomater Res. 2025 Apr 14;29:0160. doi: 10.34133/bmr.0160. eCollection 2025. Biomater Res. 2025. PMID: 40231207 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

