Mitochondrial Neurogastrointestinal Encephalomyopathy Caused by Thymidine Phosphorylase Enzyme Deficiency: From Pathogenesis to Emerging Therapeutic Options
- PMID: 28261062
- PMCID: PMC5309216
- DOI: 10.3389/fncel.2017.00031
Mitochondrial Neurogastrointestinal Encephalomyopathy Caused by Thymidine Phosphorylase Enzyme Deficiency: From Pathogenesis to Emerging Therapeutic Options
Abstract
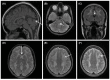
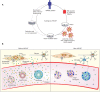
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a progressive metabolic disorder caused by thymidine phosphorylase (TP) enzyme deficiency. The lack of TP results in systemic accumulation of deoxyribonucleosides thymidine (dThd) and deoxyuridine (dUrd). In these patients, clinical features include mental regression, ophthalmoplegia, and fatal gastrointestinal complications. The accumulation of nucleosides also causes imbalances in mitochondrial DNA (mtDNA) deoxyribonucleoside triphosphates (dNTPs), which may play a direct or indirect role in the mtDNA depletion/deletion abnormalities, although the exact underlying mechanism remains unknown. The available therapeutic approaches include dialysis and enzyme replacement therapy, both can only transiently reverse the biochemical imbalance. Allogeneic hematopoietic stem cell transplantation is shown to be able to restore normal enzyme activity and improve clinical manifestations in MNGIE patients. However, transplant related complications and disease progression result in a high mortality rate. New therapeutic approaches, such as adeno-associated viral vector and hematopoietic stem cell gene therapy have been tested in Tymp-/-Upp1-/- mice, a murine model for MNGIE. This review provides background information on disease manifestations of MNGIE with a focus on current management and treatment options. It also outlines the pre-clinical approaches toward future treatment of the disease.
Keywords: HSCGT; HSCT; MNGIE; lentiviral vector; metabolic disease; mitochondrial neurogastrointestinal encephalomyopathy; thymidine phosphorylase.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

