Ginsenoside Rg5 Inhibits Succinate-Associated Lipolysis in Adipose Tissue and Prevents Muscle Insulin Resistance
- PMID: 28261091
- PMCID: PMC5306250
- DOI: 10.3389/fphar.2017.00043
Ginsenoside Rg5 Inhibits Succinate-Associated Lipolysis in Adipose Tissue and Prevents Muscle Insulin Resistance
Abstract
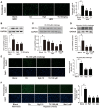
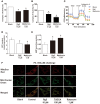
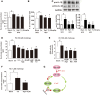
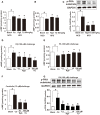
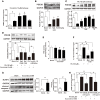
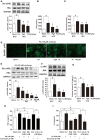
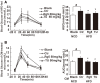
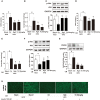
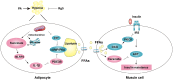
Endoplasmic reticulum (ER) stress, inflammation, and lipolysis occur simultaneously in adipose dysfunction and contribute to insulin resistance. This study was designed to investigate whether ginsenoside Rg5 could ameliorate adipose dysfunction and prevent muscle insulin resistance. Short-term high-fat diet (HFD) feeding induced hypoxia with ER stress in adipose tissue, leading to succinate accumulation due to the reversal of succinate dehydrogenase (SDH) activity. Rg5 treatment reduced cellular energy charge, suppressed ER stress and then prevented succinate accumulation in adipose tissue. Succinate promoted IL-1β production through NLRP3 inflammasome activation and then increased cAMP accumulation by impairing PDE3B expression, leading to increased lipolysis. Ginsenoside Rg5 treatment suppressed NLRP3 inflammasome activation, preserved PDE3B expression and then reduced cAMP accumulation, contributing to inhibition of lipolysis. Adipose lipolysis increased FFAs trafficking from adipose tissue to muscle. Rg5 reduced diacylglycerol (DAG) and ceramides accumulation, inhibited protein kinase Cθ translocation, and prevented insulin resistance in muscle. In conclusion, succinate accumulation in hypoxic adipose tissue acts as a metabolic signaling to link ER stress, inflammation and cAMP/PKA activation, contributing to lipolysis and insulin resistance. These findings establish a previously unrecognized role of ginsenosides in the regulation of lipid and glucose homeostasis and suggest that adipose succinate-associated NLRP3 inflammasome activation might be targeted therapeutically to prevent lipolysis and insulin resistance.
Keywords: ER stress; Ginsenoside Rg5; Lipolysis; hypoxia; insulin resistance; succinate.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

