Endocannabinoids: A Promising Impact for Traumatic Brain Injury
- PMID: 28261100
- PMCID: PMC5314139
- DOI: 10.3389/fphar.2017.00069
Endocannabinoids: A Promising Impact for Traumatic Brain Injury
Abstract
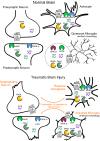
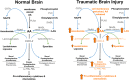
The endogenous cannabinoid (endocannabinoid) system regulates a diverse array of physiological processes and unsurprisingly possesses considerable potential targets for the potential treatment of numerous disease states, including two receptors (i.e., CB1 and CB2 receptors) and enzymes regulating their endogenous ligands N-arachidonoylethanolamine (anandamide) and 2-arachidonyl glycerol (2-AG). Increases in brain levels of endocannabinoids to pathogenic events suggest this system plays a role in compensatory repair mechanisms. Traumatic brain injury (TBI) pathology remains mostly refractory to currently available drugs, perhaps due to its heterogeneous nature in etiology, clinical presentation, and severity. Here, we review pre-clinical studies assessing the therapeutic potential of cannabinoids and manipulations of the endocannabinoid system to ameliorate TBI pathology. Specifically, manipulations of endocannabinoid degradative enzymes (e.g., fatty acid amide hydrolase, monoacylglycerol lipase, and α/β-hydrolase domain-6), CB1 and CB2 receptors, and their endogenous ligands have shown promise in modulating cellular and molecular hallmarks of TBI pathology such as; cell death, excitotoxicity, neuroinflammation, cerebrovascular breakdown, and cell structure and remodeling. TBI-induced behavioral deficits, such as learning and memory, neurological motor impairments, post-traumatic convulsions or seizures, and anxiety also respond to manipulations of the endocannabinoid system. As such, the endocannabinoid system possesses potential drugable receptor and enzyme targets for the treatment of diverse TBI pathology. Yet, full characterization of TBI-induced changes in endocannabinoid ligands, enzymes, and receptor populations will be important to understand that role this system plays in TBI pathology. Promising classes of compounds, such as the plant-derived phytocannabinoids, synthetic cannabinoids, and endocannabinoids, as well as their non-cannabinoid receptor targets, such as TRPV1 receptors, represent important areas of basic research and potential therapeutic interest to treat TBI.
Keywords: cannabinoid; endocannabinoid; neuroprotection; phytocannabinoid; traumatic brain injury.
Figures
Similar articles
-
Altered endocannabinoid metabolism compromises the brain-CSF barrier and exacerbates chronic deficits after traumatic brain injury in mice.Exp Neurol. 2023 Mar;361:114320. doi: 10.1016/j.expneurol.2023.114320. Epub 2023 Jan 7. Exp Neurol. 2023. PMID: 36627040 Free PMC article.
-
The Endocannabinoid System Modulating Levels of Consciousness, Emotions and Likely Dream Contents.CNS Neurol Disord Drug Targets. 2017;16(4):370-379. doi: 10.2174/1871527316666170223161908. CNS Neurol Disord Drug Targets. 2017. PMID: 28240187 Review.
-
Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment.Pharmacol Res. 2013 Jan;67(1):94-109. doi: 10.1016/j.phrs.2012.10.013. Epub 2012 Nov 2. Pharmacol Res. 2013. PMID: 23127915 Free PMC article.
-
Untapped endocannabinoid pharmacological targets: Pipe dream or pipeline?Pharmacol Biochem Behav. 2021 Jul;206:173192. doi: 10.1016/j.pbb.2021.173192. Epub 2021 Apr 29. Pharmacol Biochem Behav. 2021. PMID: 33932409 Review.
-
Granulocyte Colony-Stimulating Factor Enhances Brain Repair Following Traumatic Brain Injury Without Requiring Activation of Cannabinoid Receptors.Cannabis Cannabinoid Res. 2021 Feb 12;6(1):48-57. doi: 10.1089/can.2019.0090. eCollection 2021. Cannabis Cannabinoid Res. 2021. PMID: 33614952 Free PMC article.
Cited by
-
Low brain endocannabinoids associated with persistent non-goal directed nighttime hyperactivity after traumatic brain injury in mice.Sci Rep. 2020 Sep 10;10(1):14929. doi: 10.1038/s41598-020-71879-x. Sci Rep. 2020. PMID: 32913220 Free PMC article.
-
Possible Association of Nucleobindin-1 Protein with Depressive Disorder in Patients with HIV Infection.Brain Sci. 2022 Aug 29;12(9):1151. doi: 10.3390/brainsci12091151. Brain Sci. 2022. PMID: 36138887 Free PMC article.
-
Neurotransmitter changes after traumatic brain injury: an update for new treatment strategies.Mol Psychiatry. 2019 Jul;24(7):995-1012. doi: 10.1038/s41380-018-0239-6. Epub 2018 Sep 13. Mol Psychiatry. 2019. PMID: 30214042 Review.
-
Cannabinoid Drugs-Related Neuroprotection as a Potential Therapeutic Tool Against Chemotherapy-Induced Cognitive Impairment.Front Pharmacol. 2021 Nov 12;12:734613. doi: 10.3389/fphar.2021.734613. eCollection 2021. Front Pharmacol. 2021. PMID: 34867342 Free PMC article. Review.
-
Review of the neurological benefits of phytocannabinoids.Surg Neurol Int. 2018 Apr 26;9:91. doi: 10.4103/sni.sni_45_18. eCollection 2018. Surg Neurol Int. 2018. PMID: 29770251 Free PMC article. Review.
References
-
- Alvarez J., Quevedo O., Furelos L., Gonzalez I., Llapur E., Valeron M., et al. (2015). Pulmonary complications in patients with severe brain injury. Pulm. Res. Respir. Med. 2 69–74. 10.17140/PRRMOJ-2-110 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

