Aestivation Induces Changes in the mRNA Expression Levels and Protein Abundance of Two Isoforms of Urea Transporters in the Gills of the African Lungfish, Protopterus annectens
- PMID: 28261105
- PMCID: PMC5311045
- DOI: 10.3389/fphys.2017.00071
Aestivation Induces Changes in the mRNA Expression Levels and Protein Abundance of Two Isoforms of Urea Transporters in the Gills of the African Lungfish, Protopterus annectens
Abstract
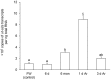
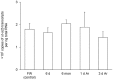
The African lungfish, Protopterus annectens, is ammonotelic in water despite being ureogenic. When it aestivates in mucus cocoon on land, ammonia is detoxified to urea. During the maintenance phase of aestivation, urea accumulates in the body, which is subsequently excreted upon arousal. Urea excretion involves urea transporters (UT/Ut). This study aimed to clone and sequence the ut isoforms from the gills of P. annectens, and to test the hypothesis that the mRNA and/or protein expression levels of ut/Ut isoforms could vary in the gills of P. annectens during the induction, maintenance, and arousal phases of aestivation. Two isoforms of ut, ut-a2a and ut-a2b, were obtained from the gills of P. annectens. ut-a2a consisted of 1227 bp and coded for 408 amino acids with an estimated molecular mass of 44.7 kDa, while ut-a2b consisted of 1392 bp and coded for 464 amino acids with an estimated molecular mass of 51.2 kDa. Ut-a2a and Ut-a2b of P. annectens had a closer phylogenetic relationship with Ut/UT of tetrapods than Ut of fishes. While the mRNA expression pattern of ut-a2a and ut-a2b across various tissues of P. annectens differed, the transcript levels of ut-a2a and ut-a2b in the gills were comparable, indicating that they might be equally important for branchial urea excretion during the initial arousal phase of aestivation. During the maintenance phase of aestivation, the transcript level of ut-a2a increased significantly, but the protein abundance of Ut-a2a remained unchanged in the gills of P. annectens. This could be an adaptive feature to prepare for an increase in the production of Ut-a2a upon arousal. Indeed, arousal led to a significant increase in the branchial Ut-a2a protein abundance. Although the transcript level of ut-a2b remained unchanged, there were significant increases in the protein abundance of Ut-a2b in the gills of P. annectens throughout the three phases of aestivation. The increase in the protein abundance of Ut-a2b during the maintenance phase could also be an adaptive feature to prepare for efficient urea excretion when water becomes available.
Keywords: Ut-a2a; Ut-a2b; ammonia; nitrogenous waste; urea excretion.
Figures





Similar articles
-
Molecular characterization of three Rhesus glycoproteins from the gills of the African lungfish, Protopterus annectens, and effects of aestivation on their mRNA expression levels and protein abundance.PLoS One. 2017 Oct 26;12(10):e0185814. doi: 10.1371/journal.pone.0185814. eCollection 2017. PLoS One. 2017. PMID: 29073147 Free PMC article.
-
Molecular Characterization of Aquaporin 1 and Aquaporin 3 from the Gills of the African Lungfish, Protopterus annectens, and Changes in Their Branchial mRNA Expression Levels and Protein Abundance during Three Phases of Aestivation.Front Physiol. 2016 Nov 10;7:532. doi: 10.3389/fphys.2016.00532. eCollection 2016. Front Physiol. 2016. PMID: 27891097 Free PMC article.
-
Brain Na+/K+-ATPase α-subunit isoforms and aestivation in the African lungfish, Protopterus annectens.J Comp Physiol B. 2014 Jul;184(5):571-87. doi: 10.1007/s00360-014-0809-0. Epub 2014 Apr 3. J Comp Physiol B. 2014. PMID: 24696295
-
Nitrogen metabolism and excretion during aestivation.Prog Mol Subcell Biol. 2010;49:63-94. doi: 10.1007/978-3-642-02421-4_4. Prog Mol Subcell Biol. 2010. PMID: 20069405 Review.
-
Ammonia excretion and urea handling by fish gills: present understanding and future research challenges.J Exp Zool. 2002 Aug 1;293(3):284-301. doi: 10.1002/jez.10123. J Exp Zool. 2002. PMID: 12115902 Review.
Cited by
-
Sodium Handling and Interaction in Numerous Organs.Am J Hypertens. 2020 Aug 4;33(8):687-694. doi: 10.1093/ajh/hpaa049. Am J Hypertens. 2020. PMID: 32198504 Free PMC article. Review.
-
Effects of Experimental Terrestrialization on the Skin Mucus Proteome of African Lungfish (Protopterus dolloi).Front Immunol. 2018 Jun 4;9:1259. doi: 10.3389/fimmu.2018.01259. eCollection 2018. Front Immunol. 2018. PMID: 29915597 Free PMC article.
-
Comparative transcriptomic evidence of physiological changes and potential relationships in vertebrates under different dormancy states.Zool Res. 2024 Mar 18;45(2):341-354. doi: 10.24272/j.issn.2095-8137.2023.308. Zool Res. 2024. PMID: 38485504 Free PMC article.
-
Two novel, tightly linked, and rapidly evolving genes underlie Aedes aegypti mosquito reproductive resilience during drought.Elife. 2023 Feb 6;12:e80489. doi: 10.7554/eLife.80489. Elife. 2023. PMID: 36744865 Free PMC article.
-
A single-cell atlas of West African lungfish respiratory system reveals evolutionary adaptations to terrestrialization.Nat Commun. 2023 Sep 13;14(1):5630. doi: 10.1038/s41467-023-41309-3. Nat Commun. 2023. PMID: 37699889 Free PMC article.
References
-
- Ballantyne J. S., Frick N. T. (2010). Lungfish metabolism, in The Biology of Lungfishes, eds Jorgensen J. M., Joss J. (Enfield, NH: Science Publishers; ), 301–335.
-
- Bradford A. D., Terris J. M., Ecelbarger C. A., Klein J. D., Sands J. M., Chou C. L., et al. (2001). 97-and 117-kDa forms of collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am. J. Physiol. Renal Physiol. 281, F133–F143. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

