Low-Frequency Oscillations and Control of the Motor Output
- PMID: 28261107
- PMCID: PMC5306248
- DOI: 10.3389/fphys.2017.00078
Low-Frequency Oscillations and Control of the Motor Output
Abstract
A less precise force output impairs our ability to perform movements, learn new motor tasks, and use tools. Here we show that low-frequency oscillations in force are detrimental to force precision. We summarize the recent evidence that low-frequency oscillations in force output represent oscillations of the spinal motor neuron pool from the voluntary drive, and can be modulated by shifting power to higher frequencies. Further, force oscillations below 0.5 Hz impair force precision with increased voluntary drive, aging, and neurological disease. We argue that the low-frequency oscillations are (1) embedded in the descending drive as shown by the activation of multiple spinal motor neurons, (2) are altered with force intensity and brain pathology, and (3) can be modulated by visual feedback and motor training to enhance force precision. Thus, low-frequency oscillations in force provide insight into how the human brain regulates force precision.
Keywords: corticomuscular coherence; force precision; force variability; motor control; voluntary drive.
Figures

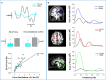
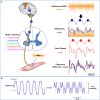
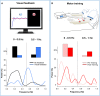
References
-
- Bernstein N. (1967). The Coordination and Regulation of Movements. Oxford: Pergamon Press.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

