The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report
- PMID: 28261109
- PMCID: PMC5310167
- DOI: 10.3389/fphys.2017.00087
The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report
Abstract
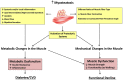
A growing body of scientific literature suggests that not only changes in skeletal muscle mass, but also other factors underpinning muscle quality, play a role in the decline in skeletal muscle function and impaired mobility associated with aging. A symposium on muscle quality and the need for standardized assessment was held on April 28, 2016 at the International Conference on Frailty and Sarcopenia Research in Philadelphia, Pennsylvania. The purpose of this symposium was to provide a venue for basic science and clinical researchers and expert clinicians to discuss muscle quality in the context of skeletal muscle function deficit and other aging-related muscle dysfunctions. The present article provides an expanded introduction concerning the emerging definitions of muscle quality and a potential framework for scientific inquiry within the field. Changes in muscle tissue composition, based on excessive levels of inter- and intra-muscular adipose tissue and intramyocellular lipids, have been found to adversely impact metabolism and peak force generation. However, methods to easily and rapidly assess muscle tissue composition in multiple clinical settings and with minimal patient burden are needed. Diagnostic ultrasound and other assessment methods continue to be developed for characterizing muscle pathology, and enhanced sonography using sensors to provide user feedback and improve reliability is currently the subject of ongoing investigation and development. In addition, measures of relative muscle force such as specific force or grip strength adjusted for body size have been proposed as methods to assess changes in muscle quality. Furthermore, performance-based assessments of muscle power via timed tests of function and body size estimates, are associated with lower extremity muscle strength may be responsive to age-related changes in muscle quality. Future aims include reaching consensus on the definition and standardized assessments of muscle quality, and providing recommendations to address critical clinical and technology research gaps within the field.
Keywords: imaging; muscle power; muscle quality; muscle strength; myosteatosis; sarcopenia; skeletal muscle function deficit.
Figures

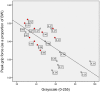
 ) represent study participants with Class I sarcopenia (5.76–6.75 kg/m2) based on lean body mass estimates from DXA scanning, and the clear data markers (◦) represent study participants with normal body mass (>6.75 kg/m2). BW, body weight; scaled peak grip force, peak force in kg/body weight in kg; both scaled peak force and grayscale values are unitless measures.
) represent study participants with Class I sarcopenia (5.76–6.75 kg/m2) based on lean body mass estimates from DXA scanning, and the clear data markers (◦) represent study participants with normal body mass (>6.75 kg/m2). BW, body weight; scaled peak grip force, peak force in kg/body weight in kg; both scaled peak force and grayscale values are unitless measures.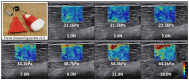
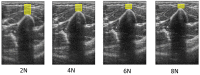
References
-
- Albu J. B., Kovera A. J., Allen L., Wainwright M., Berk E., Raja-Khan N., et al. (2005). Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am. J. Clin. Nutr. 82, 1210–1217. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

