Immune Regulation of sickle Cell Alloimmunization
- PMID: 28261322
- PMCID: PMC5330297
- DOI: 10.1111/voxs.12296
Immune Regulation of sickle Cell Alloimmunization
Abstract
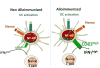
Red blood cell (RBC) transfusion remains an important treatment for patients with sickle cell disease (SCD) and the majority of patients receive transfusions by adulthood. However, SCD patients are at a high risk of alloimmunization, which can cause life-threatening complications. The high rate of alloimmunization can in part be explained by chronic inflammatory condition in SCD characterized by significant immune and inflammatory activation. Heightened immune effector cell responses and/or impaired regulatory networks are likely to drive alloantibody production in alloimmunized SCD patients. In support of this, altered T cell immunoregulation, known to control antibody responses, have been reported in alloimmunized SCD patients. In addition, stronger follicular help T cell responses that help antibody production by B cells were described in alloimmunized as compared to non-alloimmunized SCD patients. Furthermore, several innate immune abnormalities have been identified in alloimmunized SCD patients, including a compromised anti-inflammatory response against extracellular cell free heme. The data support a model in which alloimmunized SCD patients are unable to switch off their proinflammatory state in response to the ongoing hemolytic state characteristic of SCD, placing this patient subset at a higher risk to develop a strong immune response against allogeneic determinants on transfused RBCs, thus increasing the risk of further alloimmunization. A detailed mechanistic understanding of innate immune abnormalities that can contribute to pathogenic T cell responses in alloimmunized SCD patients will lay the foundation for identification of biomarkers of alloimmunization with the goal that this information will ultimately help guide therapy in these patients.
Keywords: CD83; Follicular helper T cells; NFκB; TIGIT; Tregs; alloimmunization; dendritic cells; heme oxygenase; hemolysis; sickle cell.
Conflict of interest statement
Disclosure of interest The authors have no conflicts of interest concerning this article.
Figures
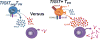
Similar articles
-
Immunoregulatory networks in sickle cell alloimmunization.Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):457-461. doi: 10.1182/asheducation-2016.1.457. Hematology Am Soc Hematol Educ Program. 2016. PMID: 27913516 Free PMC article. Review.
-
Hemin controls T cell polarization in sickle cell alloimmunization.J Immunol. 2014 Jul 1;193(1):102-10. doi: 10.4049/jimmunol.1400105. Epub 2014 May 30. J Immunol. 2014. PMID: 24879794 Free PMC article. Clinical Trial.
-
Mechanisms of sickle cell alloimmunization.Transfus Clin Biol. 2015 Aug;22(3):178-81. doi: 10.1016/j.tracli.2015.05.005. Epub 2015 Jun 6. Transfus Clin Biol. 2015. PMID: 26056038 Free PMC article. Review.
-
Altered heme-mediated modulation of dendritic cell function in sickle cell alloimmunization.Haematologica. 2016 Sep;101(9):1028-38. doi: 10.3324/haematol.2016.147181. Epub 2016 May 26. Haematologica. 2016. PMID: 27229712 Free PMC article.
-
Hemolysis inhibits humoral B-cell responses and modulates alloimmunization risk in patients with sickle cell disease.Blood. 2021 Jan 14;137(2):269-280. doi: 10.1182/blood.2020008511. Blood. 2021. PMID: 33152749 Free PMC article.
Cited by
-
Two Consecutive Episodes of Severe Delayed Hemolytic Transfusion Reaction in a Sickle Cell Disease Patient.Case Rep Hematol. 2020 Apr 14;2020:2765012. doi: 10.1155/2020/2765012. eCollection 2020. Case Rep Hematol. 2020. PMID: 32351743 Free PMC article.
-
Variant RHD alleles and Rh immunization in patients with sickle cell disease.Br J Haematol. 2023 Jun;201(6):1220-1228. doi: 10.1111/bjh.18774. Epub 2023 Apr 1. Br J Haematol. 2023. PMID: 37002797 Free PMC article.
-
Frequency of red blood cell allo-immunization in transfused patients with sickle cell disease in Africa: a systematic review with meta-analysis.Afr Health Sci. 2024 Sep;24(3):417-429. doi: 10.4314/ahs.v24i3.46. Afr Health Sci. 2024. PMID: 40777935 Free PMC article. Review.
-
Red blood cell alloimmunization and sickle cell disease: a narrative review on antibody induction.Ann Blood. 2020 Dec;5:33. doi: 10.21037/aob-2020-scd-01. Epub 2020 Dec 30. Ann Blood. 2020. PMID: 33554044 Free PMC article.
References
-
- Vichinsky EP. Current issues with blood transfusions in sickle cell disease. Semin Hematol. 2001;38:14–22. - PubMed
-
- Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. Blood. 1990;76:1431–1437. - PubMed
-
- Gupta R, Singh DK, Singh B, Rusia U. Alloimmunization to red cells in thalassemics: emerging problem and future strategies. Transfus Apher Sci. 2011;45:167–170. - PubMed
-
- Chou ST, Jackson T, Vege S, et al. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
