Human Salivary Protein Histatin 5 Has Potent Bactericidal Activity against ESKAPE Pathogens
- PMID: 28261570
- PMCID: PMC5309243
- DOI: 10.3389/fcimb.2017.00041
Human Salivary Protein Histatin 5 Has Potent Bactericidal Activity against ESKAPE Pathogens
Abstract
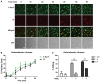
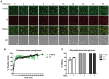
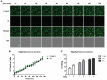
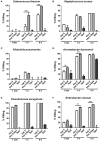
ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumanni, Pseudomonas aeruginosa, and Enterobacter species) pathogens have characteristic multiple-drug resistance and cause an increasing number of nosocomial infections worldwide. Peptide-based therapeutics to treat ESKAPE infections might be an alternative to conventional antibiotics. Histatin 5 (Hst 5) is a salivary cationic histidine-rich peptide produced only in humans and higher primates. It has high antifungal activity against Candida albicans through an energy-dependent, non-lytic process; but its bactericidal effects are less known. We found Hst 5 has bactericidal activity against S. aureus (60-70% killing) and A. baumannii (85-90% killing) in 10 and 100 mM sodium phosphate buffer (NaPB), while killing of >99% of P. aeruginosa, 60-80% E. cloacae and 20-60% of E. faecium was found in 10 mM NaPB. Hst 5 killed 60% of biofilm cells of P. aeruginosa, but had reduced activity against biofilms of S. aureus and A. baumannii. Hst 5 killed 20% of K. pneumonia biofilm cells but not planktonic cells. Binding and uptake studies using FITC-labeled Hst 5 showed E. faecium and E. cloacae killing required Hst 5 internalization and was energy dependent, while bactericidal activity was rapid against P. aeruginosa and A. baumannii suggesting membrane disruption. Hst 5-mediated killing of S. aureus was both non-lytic and energy independent. Additionally, we found that spermidine conjugated Hst 5 (Hst5-Spd) had improved killing activity against E. faecium, E. cloacae, and A. baumannii. Hst 5 or its derivative has antibacterial activity against five out of six ESKAPE pathogens and may be an alternative treatment for these infections.
Keywords: Candida albicans; ESKAPE; Histatin 5; antimicrobial peptide; bactericidal activity.
Figures




Similar articles
-
Bactericidal efficacy of atmospheric pressure non-thermal plasma (APNTP) against the ESKAPE pathogens.Int J Antimicrob Agents. 2015 Jul;46(1):101-7. doi: 10.1016/j.ijantimicag.2015.02.026. Epub 2015 Apr 20. Int J Antimicrob Agents. 2015. PMID: 25963338
-
Expanding the potential of NAI-107 for treating serious ESKAPE pathogens: synergistic combinations against Gram-negatives and bactericidal activity against non-dividing cells.J Antimicrob Chemother. 2018 Feb 1;73(2):414-424. doi: 10.1093/jac/dkx395. J Antimicrob Chemother. 2018. PMID: 29092042 Free PMC article.
-
Norfloxacin salts of carboxylic acids curtail planktonic and biofilm mode of growth in ESKAPE pathogens.J Appl Microbiol. 2018 Feb;124(2):408-422. doi: 10.1111/jam.13651. J Appl Microbiol. 2018. PMID: 29178633
-
Clinical relevance of the ESKAPE pathogens.Expert Rev Anti Infect Ther. 2013 Mar;11(3):297-308. doi: 10.1586/eri.13.12. Expert Rev Anti Infect Ther. 2013. PMID: 23458769 Review.
-
Ventilator-associated pneumonia caused by ESKAPE organisms: cause, clinical features, and management.Curr Opin Pulm Med. 2012 May;18(3):187-93. doi: 10.1097/MCP.0b013e328351f974. Curr Opin Pulm Med. 2012. PMID: 22366995 Review.
Cited by
-
Top-Down Proteomics of Human Saliva Discloses Significant Variations of the Protein Profile in Patients with Mastocytosis.J Proteome Res. 2020 Aug 7;19(8):3238-3253. doi: 10.1021/acs.jproteome.0c00207. Epub 2020 Jul 6. J Proteome Res. 2020. PMID: 32575983 Free PMC article.
-
Are Antimicrobial Peptide Dendrimers an Escape from ESKAPE?Adv Wound Care (New Rochelle). 2020 May 19;9(7):378-95. doi: 10.1089/wound.2019.1113. Online ahead of print. Adv Wound Care (New Rochelle). 2020. PMID: 32320368 Free PMC article.
-
Silver nanoparticles synthesized from Pseudomonas aeruginosa pyoverdine: Antibiofilm and antivirulence agents.Biofilm. 2024 Mar 15;7:100192. doi: 10.1016/j.bioflm.2024.100192. eCollection 2024 Jun. Biofilm. 2024. PMID: 38544742 Free PMC article.
-
Histatins, proangiogenic molecules with therapeutic implications in regenerative medicine.iScience. 2024 Nov 1;27(12):111309. doi: 10.1016/j.isci.2024.111309. eCollection 2024 Dec 20. iScience. 2024. PMID: 39634559 Free PMC article. Review.
-
Water-Mediated Dissemination and Detection of Antibiotic Resistance Across Livestock, Agri-Food, and Aquaculture Systems.Micromachines (Basel). 2025 Aug 13;16(8):934. doi: 10.3390/mi16080934. Micromachines (Basel). 2025. PMID: 40872439 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

