Effect of triptorelin on lower urinary tract symptoms in Australian prostate cancer patients
- PMID: 28261572
- PMCID: PMC5328125
- DOI: 10.2147/RRU.S125791
Effect of triptorelin on lower urinary tract symptoms in Australian prostate cancer patients
Abstract
Purpose: Prostate cancer is often comorbidly associated with lower urinary tract symptoms (LUTS), but few studies have assessed the effects of androgen deprivation therapy on LUTS in this patient group.
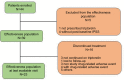
Patients and methods: We conducted a prospective, noninterventional, multicenter, observational study to assess the effectiveness of triptorelin (11.25 mg every 12 weeks) over 48 weeks in men presenting with local stage T3/4 prostate cancer and moderate to severe LUTS (International Prostate Symptom Score [IPSS] >7) in a routine practice setting in Australia.
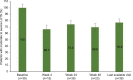
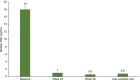
Results: Of the 44 men who enrolled, effectiveness data were available for 39 men. By the end of the study, 30% of men no longer met the IPSS criteria for moderate to severe LUTS. The proportion of patients with moderate to severe LUTS was 69.6% (16/23) at week 48 and 76.9% (30/39) at the last available visit (coprimary outcomes). An IPSS reduction of ≥3 from week 0 was observed in 47% of men at week 4, 56% at week 24, 61% (14/23) at week 48, and 61.5% (24/39) at the last available visit. Quality of life was rated as mostly satisfied-to-delighted by 39.5% of patients at week 0, 53.9% at week 24, and 77.3% at week 48. Triptorelin was well tolerated with 8 treatment-related adverse events reported, half of which were hot flushes; 5 patients discontinued due to the reported treatment-related adverse events.
Conclusion: This observational study suggests that triptorelin improves moderate to severe LUTS in prostate cancer patients in a routine clinical practice setting.
Keywords: GnRH agonist; IPSS.
Conflict of interest statement
Disclosure DGM has received honoraria for speaking and consulting services from Ipsen Pty Ltd. JPG has received a research grant and honoraria for consulting services from Ipsen Pty Ltd. APS is an employee of Ipsen Pty Ltd. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Triptorelin therapy for lower urinary tract symptoms (LUTS) in prostate cancer patients: A systematic meta-analysis.BJUI Compass. 2023 Oct 10;5(1):17-28. doi: 10.1002/bco2.292. eCollection 2024 Jan. BJUI Compass. 2023. PMID: 38179030 Free PMC article. Review.
-
Triptorelin relieves lower urinary tract symptoms in Chinese advanced prostate cancer patients: a multicenter, non-interventional, prospective study.BMC Urol. 2018 Mar 27;18(1):23. doi: 10.1186/s12894-018-0337-4. BMC Urol. 2018. PMID: 29587718 Free PMC article.
-
A prospective, observational grouped analysis to evaluate the effect of triptorelin on lower urinary tract symptoms in patients with advanced prostate cancer.Ther Adv Urol. 2015 Jun;7(3):116-24. doi: 10.1177/1756287215574480. Ther Adv Urol. 2015. PMID: 26161142 Free PMC article.
-
[Study of the beneficial effects of triptorelin on lower urinary tract symptoms in Algeria in patients with non-localized prostate cancer].Prog Urol. 2018 Jun;28(8-9):450-459. doi: 10.1016/j.purol.2018.03.014. Epub 2018 May 20. Prog Urol. 2018. PMID: 29789236 French.
-
Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia.BJU Int. 2019 Jul;124(1):27-34. doi: 10.1111/bju.14689. Epub 2019 Mar 11. BJU Int. 2019. PMID: 30681264
Cited by
-
Triptorelin therapy for lower urinary tract symptoms (LUTS) in prostate cancer patients: A systematic meta-analysis.BJUI Compass. 2023 Oct 10;5(1):17-28. doi: 10.1002/bco2.292. eCollection 2024 Jan. BJUI Compass. 2023. PMID: 38179030 Free PMC article. Review.
-
Subsequent risk of acute urinary retention and androgen deprivation therapy in patients with prostate cancer: A population-based retrospective cohort study.Medicine (Baltimore). 2020 Feb;99(7):e18842. doi: 10.1097/MD.0000000000018842. Medicine (Baltimore). 2020. PMID: 32049786 Free PMC article.
-
Effect of LHRH analogs on lower urinary tract symptoms associated with advanced prostate cancer in real clinical practice: ANALUTS study.Neurourol Urodyn. 2022 Nov;41(8):1824-1833. doi: 10.1002/nau.25031. Epub 2022 Sep 7. Neurourol Urodyn. 2022. PMID: 36069170 Free PMC article.
-
Triptorelin relieves lower urinary tract symptoms in Chinese advanced prostate cancer patients: a multicenter, non-interventional, prospective study.BMC Urol. 2018 Mar 27;18(1):23. doi: 10.1186/s12894-018-0337-4. BMC Urol. 2018. PMID: 29587718 Free PMC article.
-
MCM3AP-AS1 KD Inhibits Proliferation, Invasion, and Migration of PCa Cells via DNMT1/DNMT3 (A/B) Methylation-Mediated Upregulation of NPY1R.Mol Ther Nucleic Acids. 2020 Jun 5;20:265-278. doi: 10.1016/j.omtn.2020.01.016. Epub 2020 Jan 23. Mol Ther Nucleic Acids. 2020. Retraction in: Mol Ther Nucleic Acids. 2022 Apr 05;28:279. doi: 10.1016/j.omtn.2022.03.026. PMID: 32193153 Free PMC article. Retracted.
References
-
- Labrie F, Belanger A, Luu-The V, et al. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev. 2005;26(3):361–379. - PubMed
-
- Lundstrom EA, Rencken RK, van Wyk JH, et al. Triptorelin 6-month formulation in the management of patients with locally advanced and metastatic prostate cancer: an open-label, non-comparative, multicentre, phase III study. Clin Drug Investig. 2009;29(12):757–765. - PubMed
-
- Andersson SO, Rashidkhani B, Karlberg L, Wolk A, Johansson JE. Prevalence of lower urinary tract symptoms in men aged 45–79 years: a population-based study of 40 000 Swedish men. BJU Int. 2004;94(3):327–331. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

