Safety and Efficacy of A High Performance Graphene-Based Magnetic Resonance Imaging Contrast Agent for Renal Abnormalities
- PMID: 28261636
- PMCID: PMC5333926
- DOI: 10.1007/s41127-016-0001-2
Safety and Efficacy of A High Performance Graphene-Based Magnetic Resonance Imaging Contrast Agent for Renal Abnormalities
Abstract
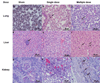
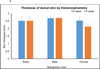
The etiology of renal insufficiency includes primary (e.g polycystic kidney disease) or secondary (e.g. contrast media, diabetes) causes. The regulatory restrictions placed on the use of contrast agents (CAs) for non-invasive imaging modalities such as X-ray computed tomography (CT) and magnetic resonance imaging (MRI) affects the clinical management of these patients. With the goal to develop a next-generation CA for unfettered use for renal MRI, here we report, in a rodent model of chronic kidney disease, the preclinical safety and efficacy of a novel nanoparticle CA comprising of manganese (Mn2+) ions intercalated graphene coated with dextran (hereafter called Mangradex). Nephrectomized rats received single or 5 times/week repeat (2 or 4 weeks) intravenous (IV) injections of Mangradex at two potential (low = 5 mg/kg, and high = 50 mg/kg) therapeutic doses. Histopathology results indicate that Mangradex does not elicit nephrogenic systemic fibrosis (NSF)-like indicators or questionable effects on vital organs of rodents. MRI at 7 Tesla magnetic field was performed on these rats immediately after IV injections of Mangradex at one potential therapeutic dose (25 mg/kg, [Mn2+] = 60 nmoles/kg) for 90 minutes. The results indicated significant (>100%) and sustained contrast enhancement in the kidney and renal artery at these low paramagnetic ion (Mn2+) concentration; 2 orders of magnitude lower than the paramagnetic ion concentration in a typical clinical dose of long circulating Gd3+-based MRI CA gadofosveset trisodium. The results open avenues for further development of Mangradex as a MRI CA to diagnose and monitor abnormalities in renal anatomy and vasculature.
Keywords: Chronic Kidney Disease; Contrast Agent; Gadolinium; Magnetic Resonance Imaging; Mangradex; Nephrogenic Systemic Fibrosis.
Conflict of interest statement
Conflict of Interest Disclosure: Stony Brook University, along with its researchers, has filed patents related to the technology reported in this article. If licensing or commercialization occurs, the researchers are entitled to standard royalties. Balaji Sitharaman has financial interest in Theragnostic Technologies Inc., which, however, did not support this work.
Figures






Similar articles
-
Towards An Advanced Graphene-Based Magnetic Resonance Imaging Contrast Agent: Sub-acute Toxicity and Efficacy Studies in Small Animals.Sci Rep. 2015 Dec 2;5:17182. doi: 10.1038/srep17182. Sci Rep. 2015. PMID: 26625867 Free PMC article.
-
NSF evaluation of gadolinium biodistribution in renally impaired rats: Using novel metabolic Gd2O3 nanoparticles coated with β-cyclodextrin (Gd2O3@PCD) in MR molecular imaging.Magn Reson Imaging. 2024 Apr;107:120-129. doi: 10.1016/j.mri.2024.01.003. Epub 2024 Jan 11. Magn Reson Imaging. 2024. PMID: 38215955
-
[Nephrogenic systemic fibrosis].Med Monatsschr Pharm. 2009 Oct;32(10):377-82. Med Monatsschr Pharm. 2009. PMID: 19886227 Review. German.
-
Physicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agent.Int J Nanomedicine. 2013;8:2821-33. doi: 10.2147/IJN.S47062. Epub 2013 Aug 5. Int J Nanomedicine. 2013. PMID: 23946653 Free PMC article.
-
Patient characteristics and risk factors for nephrogenic systemic fibrosis following gadolinium exposure.Semin Dial. 2008 Mar-Apr;21(2):135-9. doi: 10.1111/j.1525-139X.2007.00406.x. Epub 2008 Jan 23. Semin Dial. 2008. PMID: 18226001 Review.
References
-
- Hoste EAJ, Schurgers M. Epidemiology of acute kidney injury: How big is the problem? Critical Care Medicine. 2008;36:S146–S151. - PubMed
-
- Coresh JSESLA, et al. Prevalence of chronic kidney disease in the United States. JAMA: The Journal of the American Medical Association. 2007;298:2038–2047. - PubMed
-
- Robinson BE. Epidemiology of chronic kidney disease and anemia. Journal of the American Medical Directors Association. 2006;7:S3–S6. - PubMed
-
- Roggeri DP, Roggeri A, Salomone M. Chronic Kidney Disease: Evolution of Healthcare Costs and Resource Consumption from Predialysis to Dialysis in Piedmont Region, Italy. Advances in Nephrology. 2014;2014:6.
-
- Murphree DD, Thelen SM. Chronic kidney disease in primary care. Journal of the American Board of Family Medicine. 2010;23:542–550. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
