A Combination of Two Receptor Tyrosine Kinase Inhibitors, Canertinib and PHA665752 Compromises Ovarian Cancer Cell Growth in 3D Cell Models
- PMID: 28261654
- PMCID: PMC5315083
- DOI: 10.1007/s40487-016-0031-1
A Combination of Two Receptor Tyrosine Kinase Inhibitors, Canertinib and PHA665752 Compromises Ovarian Cancer Cell Growth in 3D Cell Models
Abstract
Introduction: Advanced ovarian cancer is often a fatal disease as chemotherapeutic drugs have limited effectiveness. Better targeted therapy is needed to improve the survival and quality of life for these women. Receptor tyrosine kinases including EGFR, Her-2 and c-Met are associated with a poor prognosis in ovarian cancer. Therefore, the co-activation of these receptors may be crucial for growth promoting activity. In this study, we explored the effect of combining two small molecule inhibitors that target the EGFR/Her-2 and c-Met receptor tyrosine kinases in two ovarian cancer cell lines. The aim of this study was to investigate the combined inhibition activity of a dual EGFR/Her-2 inhibitor (canertinib) and a c-Met inhibitor (PHA665752) in ovarian cancer cell lines in 3D cell aggregates.
Methods: OVCAR-5 and SKOV-3 ovarian cancer cell lines were cultured on a non-adherent surface to produce 3D cell clusters and aggregates. Cells were exposed to canertinib and PHA665752, both individually and in combination, for 48 h. The effect on growth, metabolism and the expression/phosphorylation of selective signaling proteins associated with EGFR, Her-2 and c-Met were investigated.
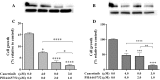
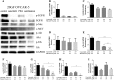
Results: The single drug treatments significantly decreased cell growth and altered the expression of signaling proteins in OVCAR-5 and SKOV-3 cell lines. The combination treatment showed greater reduction of cell numbers for both cell lines. Total expression and phosphorylation of signaling proteins were further reduced in the combination drug treatments, compared to the single inhibitor treatments.
Conclusion: Our findings suggest that the concurrent targeting of more than one receptor tyrosine kinase may be useful in developing more effective targeted drug regimens for patients, who have EGFR, Her-2 and c-Met positive ovarian cancer cells.
Keywords: Canertinib; Cell clusters; EGFR; Ovarian cancer; PHA665752; Tyrosine kinase inhibitors; c-MET.
Figures







Similar articles
-
Ascitic fluid from advanced ovarian cancer patients compromises the activity of receptor tyrosine kinase inhibitors in 3D cell clusters of ovarian cancer cells.Cancer Lett. 2018 Apr 28;420:168-181. doi: 10.1016/j.canlet.2018.02.013. Epub 2018 Feb 10. Cancer Lett. 2018. PMID: 29432847
-
Impact of the putative cancer stem cell markers and growth factor receptor expression on the sensitivity of ovarian cancer cells to treatment with various forms of small molecule tyrosine kinase inhibitors and cytotoxic drugs.Int J Oncol. 2016 Nov;49(5):1825-1838. doi: 10.3892/ijo.2016.3678. Epub 2016 Sep 5. Int J Oncol. 2016. PMID: 27599579 Free PMC article.
-
Dasatinib + Gefitinib, a non platinum-based combination with enhanced growth inhibitory, anti-migratory and anti-invasive potency against human ovarian cancer cells.J Ovarian Res. 2017 Apr 26;10(1):31. doi: 10.1186/s13048-017-0319-2. J Ovarian Res. 2017. PMID: 28446239 Free PMC article.
-
MET inhibitors in combination with other therapies in non-small cell lung cancer.Transl Lung Cancer Res. 2012 Dec;1(4):238-53. doi: 10.3978/j.issn.2218-6751.2012.10.08. Transl Lung Cancer Res. 2012. PMID: 25806189 Free PMC article. Review.
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
Cited by
-
The Anti-Proliferative Effect of PI3K/mTOR and ERK Inhibition in Monolayer and Three-Dimensional Ovarian Cancer Cell Models.Cancers (Basel). 2022 Jan 13;14(2):395. doi: 10.3390/cancers14020395. Cancers (Basel). 2022. PMID: 35053555 Free PMC article.
-
Targeted therapies in gynecological cancers: a comprehensive review of clinical evidence.Signal Transduct Target Ther. 2020 Jul 29;5(1):137. doi: 10.1038/s41392-020-0199-6. Signal Transduct Target Ther. 2020. PMID: 32728057 Free PMC article. Review.
-
Targeting c-Met in breast cancer: From mechanisms of chemoresistance to novel therapeutic strategies.Curr Res Pharmacol Drug Discov. 2024 Oct 22;7:100204. doi: 10.1016/j.crphar.2024.100204. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 39524211 Free PMC article. Review.
-
Human epidermal growth factor receptor targeted inhibitors for the treatment of ovarian cancer.Cancer Biol Med. 2018 Nov;15(4):375-388. doi: 10.20892/j.issn.2095-3941.2018.0062. Cancer Biol Med. 2018. PMID: 30766749 Free PMC article.
-
PHA-665752's Antigrowth and Proapoptotic Effects on HSC-3 Human Oral Cancer Cells.Int J Mol Sci. 2024 Mar 1;25(5):2871. doi: 10.3390/ijms25052871. Int J Mol Sci. 2024. PMID: 38474118 Free PMC article.
References
-
- Vergote IB, Jimeno A, Joly F, Katsaros D, Coens C, Despierre E, et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup Study. J Clin Oncol. 2013;32:320–326. doi: 10.1200/JCO.2013.50.5669. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

