Impact of CYP3A4*18 and CYP3A5*3 Polymorphisms on Imatinib Mesylate Response Among Chronic Myeloid Leukemia Patients in Malaysia
- PMID: 28261657
- PMCID: PMC5315081
- DOI: 10.1007/s40487-016-0035-x
Impact of CYP3A4*18 and CYP3A5*3 Polymorphisms on Imatinib Mesylate Response Among Chronic Myeloid Leukemia Patients in Malaysia
Abstract
Introduction: Imatinib mesylate (IM), a selective inhibitor of the BCR-ABL tyrosine kinase, is a well-established first-line treatment for chronic myeloid leukemia (CML). IM is metabolized mainly by cytochrome P450 (CYP) in the liver, specifically the CYP3A4 and CYP3A5 enzymes. Polymorphisms in these genes can alter the enzyme activity of IM and may affect its response. In this study, the impact of two single-nucleotide polymorphisms (SNPs), CYP3A5*3 (6986A>G) and CYP3A4*18 (878T>C), on IM treatment response in CML patients (n = 270; 139 IM resistant and 131 IM good responders) was investigated.
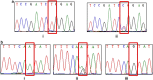
Methods: Genotyping of CYP3A4*18 and CYP3A5*3 was performed using the polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) technique. The association between allelic variants and treatment response was assessed by means of odds ratio (OR) with 95% confidence intervals calculated by logistic regression.
Results: Our results indicated that CML patients carrying the heterozygous (AG) and homozygous variant (GG) genotype of CYP3A5*3 were associated with a significantly lower risk of acquiring resistance with OR 0.171; 95% CI: 0.090-0.324, p < 0.001 and OR 0.257; 95% CI: 0.126-0.525, p < 0.001, respectively. Although CML patients carrying the heterozygous (TC) genotype of CYP3A4*18 showed a lower risk of acquiring resistance toward IM (OR 0.648; 95% CI: 0.277-1.515), the association was not statistically significant (p = 0.316). No homozygous variant (CC) genotype of CYP3A4*18 was detected among the CML patients.
Conclusion: It is concluded that polymorphism of CYP3A5*3 is associated with IM treatment response in Malaysian CML patients with carriers of CYP3A5*1/*3 and CYP3A5*3/*3 genotypes posing lower risk for development of resistance to IM.
Keywords: CYP3A4*18; CYP3A5*3; Chronic myeloid leukemia; Imatinib mesylate; Single-nucleotide polymorphisms.
Figures


Similar articles
-
Association of CYP3A5*3, CYP3A4*18 & CYP2B6*6 polymorphisms with imatinib treatment outcome in Azerbaijani chronic myeloid leukaemia patients.Indian J Med Res. 2023 Aug;158(2):151-160. doi: 10.4103/ijmr.ijmr_1103_22. Indian J Med Res. 2023. PMID: 37706370 Free PMC article.
-
Impact of Fas/Fasl Gene Polymorphisms on Susceptibility Risk and Imatinib Mesylate Treatment Response in Chronic Myeloid Leukaemia Patients.Asian Pac J Cancer Prev. 2021 Feb 1;22(2):565-571. doi: 10.31557/APJCP.2021.22.2.565. Asian Pac J Cancer Prev. 2021. PMID: 33639675 Free PMC article.
-
Do SLCO1B3 (T334G) and CYP3A5*3 polymorphisms affect response in Egyptian chronic myeloid leukemia patients receiving imatinib therapy?Hematology. 2013 Jul;18(4):211-6. doi: 10.1179/1607845412Y.0000000067. Epub 2013 Jan 31. Hematology. 2013. PMID: 23394475
-
Impact of SLC22A1 and CYP3A5 genotypes on imatinib response in chronic myeloid leukemia: A systematic review and meta-analysis.Pharmacol Res. 2018 May;131:244-254. doi: 10.1016/j.phrs.2018.02.005. Epub 2018 Feb 7. Pharmacol Res. 2018. PMID: 29427770
-
Functionally defective or altered CYP3A4 and CYP3A5 single nucleotide polymorphisms and their detection with genotyping tests.Pharmacogenomics. 2005 Jun;6(4):357-71. doi: 10.1517/14622416.6.4.357. Pharmacogenomics. 2005. PMID: 16004554 Review.
Cited by
-
Effect of Cytochrome P450 and ABCB1 Polymorphisms on Imatinib Pharmacokinetics After Single-Dose Administration to Healthy Subjects.Clin Drug Investig. 2020 Jul;40(7):617-628. doi: 10.1007/s40261-020-00921-7. Clin Drug Investig. 2020. PMID: 32415468
-
Factors Influencing the Steady-State Plasma Concentration of Imatinib Mesylate in Patients With Gastrointestinal Stromal Tumors and Chronic Myeloid Leukemia.Front Pharmacol. 2020 Nov 25;11:569843. doi: 10.3389/fphar.2020.569843. eCollection 2020. Front Pharmacol. 2020. PMID: 33381028 Free PMC article.
-
Copy number variation analysis in cytochromes and glutathione S-transferases may predict efficacy of tyrosine kinase inhibitors in chronic myeloid leukemia.PLoS One. 2017 Sep 13;12(9):e0182901. doi: 10.1371/journal.pone.0182901. eCollection 2017. PLoS One. 2017. PMID: 28902850 Free PMC article.
-
Cytochromes P450 2C8 and 3A Catalyze the Metabolic Activation of the Tyrosine Kinase Inhibitor Masitinib.Chem Res Toxicol. 2022 Sep 19;35(9):1467-1481. doi: 10.1021/acs.chemrestox.2c00057. Epub 2022 Sep 1. Chem Res Toxicol. 2022. PMID: 36048877 Free PMC article.
-
Pharmacogenetics of Drugs Used in the Treatment of Cancers.Genes (Basel). 2022 Feb 7;13(2):311. doi: 10.3390/genes13020311. Genes (Basel). 2022. PMID: 35205356 Free PMC article. Review.
References
-
- Au A, Aziz Baba A, Goh AS, Wahid Fadilah SA, Teh A, Rosline H, Ankathil R. Association of genotypes and haplotypes of multi-drug transporter genes ABCB1 and ABCG2 with clinical response to imatinib mesylate in chronic myeloid leukemia patients. Biomed Pharmacother. 2014;68(3):343–349. doi: 10.1016/j.biopha.2014.01.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

