Staurosporine Induces Filamentation in the Human Fungal Pathogen Candida albicans via Signaling through Cyr1 and Protein Kinase A
- PMID: 28261668
- PMCID: PMC5332603
- DOI: 10.1128/mSphere.00056-17
Staurosporine Induces Filamentation in the Human Fungal Pathogen Candida albicans via Signaling through Cyr1 and Protein Kinase A
Abstract
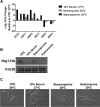
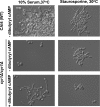
Protein kinases are key regulators of signal transduction pathways that participate in diverse cellular processes. In fungal pathogens, kinases regulate signaling pathways that govern drug resistance, stress adaptation, and pathogenesis. The impact of kinases on the fungal regulatory circuitry has recently garnered considerable attention in the opportunistic fungal pathogen Candida albicans, which is a leading cause of human morbidity and mortality. Complex regulatory circuitry governs the C. albicans morphogenetic transition between yeast and filamentous growth, which is a key virulence trait. Here, we report that staurosporine, a promiscuous kinase inhibitor that abrogates fungal drug resistance, also influences C. albicans morphogenesis by inducing filamentation in the absence of any other inducing cue. We further establish that staurosporine exerts its effect via the adenylyl cyclase Cyr1 and the cyclic AMP (cAMP)-dependent protein kinase A (PKA). Strikingly, filamentation induced by staurosporine does not require the known upstream regulators of Cyr1, Ras1 or Pkc1, or effectors downstream of PKA, including Efg1. We further demonstrate that Cyr1 is capable of activating PKA to enable filamentation in response to staurosporine through a mechanism that does not require degradation of the transcriptional repressor Nrg1. We establish that staurosporine-induced filamentation is accompanied by a defect in septin ring formation, implicating cell cycle kinases as potential staurosporine targets underpinning this cellular response. Thus, we establish staurosporine as a chemical probe to elucidate the architecture of cellular signaling governing fungal morphogenesis and highlight the existence of novel circuitry through which the Cyr1 and PKA govern a key virulence trait. IMPORTANCE The impact of fungal pathogens on human health is devastating. One of the most pervasive fungal pathogens is Candida albicans, which kills ~40% of people suffering from bloodstream infections. Treatment of these infections is extremely difficult, as fungi are closely related to humans, and there are limited drugs that kill the fungus without host toxicity. The capacity of C. albicans to transition between yeast and filamentous forms is a key virulence trait. Thus, understanding the genetic pathways that regulate morphogenesis could provide novel therapeutic targets to treat C. albicans infections. Here, we establish the small molecule staurosporine as an inducer of filamentous growth. We unveil distinct regulatory circuitry required for staurosporine-induced filamentation that appears to be unique to this filament-inducing cue. Thus, this work highlights the fact that small molecules, such as staurosporine, can improve our understanding of the pathways required for key virulence programs, which may lead to the development of novel therapeutics.
Keywords: Candida albicans; cyclic AMP; kinase inhibitor; morphogenesis; staurosporine; virulence.
Figures




Similar articles
-
The Proteasome Governs Fungal Morphogenesis via Functional Connections with Hsp90 and cAMP-Protein Kinase A Signaling.mBio. 2020 Apr 21;11(2):e00290-20. doi: 10.1128/mBio.00290-20. mBio. 2020. PMID: 32317319 Free PMC article.
-
Signaling through Lrg1, Rho1 and Pkc1 Governs Candida albicans Morphogenesis in Response to Diverse Cues.PLoS Genet. 2016 Oct 27;12(10):e1006405. doi: 10.1371/journal.pgen.1006405. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27788136 Free PMC article.
-
Candida albicans Filamentation Does Not Require the cAMP-PKA Pathway In Vivo.mBio. 2022 Jun 28;13(3):e0085122. doi: 10.1128/mbio.00851-22. Epub 2022 Apr 27. mBio. 2022. PMID: 35475642 Free PMC article.
-
Multiple roles and diverse regulation of the Ras/cAMP/protein kinase A pathway in Candida albicans.Mol Microbiol. 2019 Jan;111(1):6-16. doi: 10.1111/mmi.14148. Epub 2018 Nov 4. Mol Microbiol. 2019. PMID: 30299574 Review.
-
Functional connections between cell cycle and proteostasis in the regulation of Candida albicans morphogenesis.Cell Rep. 2021 Feb 23;34(8):108781. doi: 10.1016/j.celrep.2021.108781. Cell Rep. 2021. PMID: 33626353 Free PMC article. Review.
Cited by
-
The Proteasome Governs Fungal Morphogenesis via Functional Connections with Hsp90 and cAMP-Protein Kinase A Signaling.mBio. 2020 Apr 21;11(2):e00290-20. doi: 10.1128/mBio.00290-20. mBio. 2020. PMID: 32317319 Free PMC article.
-
Risks of Ruxolitinib in STAT1 Gain-of-Function-Associated Severe Fungal Disease.Open Forum Infect Dis. 2017 Sep 22;4(4):ofx202. doi: 10.1093/ofid/ofx202. eCollection 2017 Fall. Open Forum Infect Dis. 2017. PMID: 29226168 Free PMC article.
-
Functional divergence of a global regulatory complex governing fungal filamentation.PLoS Genet. 2019 Jan 7;15(1):e1007901. doi: 10.1371/journal.pgen.1007901. eCollection 2019 Jan. PLoS Genet. 2019. PMID: 30615616 Free PMC article.
-
Negative control of Candida albicans filamentation-associated gene expression by essential protein kinase gene KIN28.Curr Genet. 2017 Dec;63(6):1073-1079. doi: 10.1007/s00294-017-0705-8. Epub 2017 May 13. Curr Genet. 2017. PMID: 28501989 Free PMC article.
-
Duplicated ribosomal protein paralogs promote alternative translation and drug resistance.Nat Commun. 2022 Aug 23;13(1):4938. doi: 10.1038/s41467-022-32717-y. Nat Commun. 2022. PMID: 35999447 Free PMC article.
References
-
- LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AA, Perfect JR, Cowen LE. 2010. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 6:e1001069. doi:10.1371/journal.ppat.1001069. - DOI - PMC - PubMed
-
- Sussman A, Huss K, Chio LC, Heidler S, Shaw M, Ma D, Zhu G, Campbell RM, Park TS, Kulanthaivel P, Scott JE, Carpenter JW, Strege MA, Belvo MD, Swartling JR, Fischl A, Yeh WK, Shih C, Ye XS. 2004. Discovery of cercosporamide, a known antifungal natural product, as a selective Pkc1 kinase inhibitor through high-throughput screening. Eukaryot Cell 3:932–943. doi:10.1128/EC.3.4.932-943.2004. - DOI - PMC - PubMed
-
- Robbins N, Spitzer M, Wang W, Waglechner N, Patel DJ, O’Brien JS, Ejim L, Ejim O, Tyers M, Wright GD. 2016. Discovery of ibomycin, a complex macrolactone that exerts antifungal activity by impeding endocytic trafficking and membrane function. Cell Chem Biol 23:1383–1394. doi:10.1016/j.chembiol.2016.08.015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

