Dendrobium nobile Lindl alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Aβ25-35 in hippocampus neurons in vitro
- PMID: 28261990
- PMCID: PMC6492701
- DOI: 10.1111/cns.12678
Dendrobium nobile Lindl alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Aβ25-35 in hippocampus neurons in vitro
Abstract
Aims: Axonal degeneration is a pathological symbol in the early stage of Alzheimer's disease (AD), which can be triggered by amyloid-β (Aβ) peptide deposition. Growing evidence indicates that deficit of autophagy eventually leads to the axonal degeneration. Our previous studies have shown that Dendrobium nobile Lindl alkaloid (DNLA) had protective effect on neuron impairment in vivo and in vitro; however, the underlying mechanisms is still unclear.
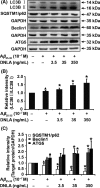
Methods: We exposed cultured hippocampus neurons to Aβ25-35 to investigate the effect of DNLA in vitro. Axonal degeneration was evaluated by immunofluorescence staining and MTT assay. Neurons overexpressing GFP-LC3B were used to measure the formation of autophagosome. Autophagosome-lysosome fusion, the lysosomal pH, and cathepsin activity were assessed to reflect autophagy process. Proteins of interest were analyzed by Western blot.
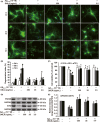
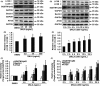
Results: DNLA pretreatment significantly inhibited axonal degeneration induced by Aβ25-35 peptide in vitro. Further studies revealed DNLA treatment increased autophagic flux through promoting formation and degradation of autophagosome in hippocampus neurons. Moreover, enhancement of autophagic flux was responsible for the protective effects of DNLA on axonal degeneration.
Conclusions: DNLA prevents Aβ25-35 -induced axonal degeneration via activation of autophagy process and could be a novel therapeutic target.
Keywords: Alzheimer's disease; Dendrobium nobile Lindl alkaloid; amyloid-β; autophagy; axonal degeneration.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dendrobium nobile Lindl. Alkaloids Ameliorate Aβ25-35-Induced Synaptic Deficits by Targeting Wnt/β-Catenin Pathway in Alzheimer's Disease Models.J Alzheimers Dis. 2022;86(1):297-313. doi: 10.3233/JAD-215433. J Alzheimers Dis. 2022. PMID: 35068466
-
Dendrobium nobile Lindl. Alkaloids Ameliorate Cognitive Dysfunction in Senescence Accelerated SAMP8 Mice by Decreasing Amyloid-β Aggregation and Enhancing Autophagy Activity.J Alzheimers Dis. 2020;76(2):657-669. doi: 10.3233/JAD-200308. J Alzheimers Dis. 2020. PMID: 32538851
-
Dendrobium nobile Lindl. alkaloids protect CCl4-induced acute liver injury via upregulating LAMP1 expression and activating autophagy flux.J Nat Med. 2025 Jan;79(1):180-195. doi: 10.1007/s11418-024-01852-9. Epub 2024 Nov 15. J Nat Med. 2025. PMID: 39546174
-
Therapeutic potential of the chemical composition of Dendrobium nobile Lindl.Front Pharmacol. 2023 Jul 11;14:1163830. doi: 10.3389/fphar.2023.1163830. eCollection 2023. Front Pharmacol. 2023. PMID: 37497110 Free PMC article. Review.
-
Neuronal autophagy and axon degeneration.Cell Mol Life Sci. 2018 Jul;75(13):2389-2406. doi: 10.1007/s00018-018-2812-1. Epub 2018 Apr 19. Cell Mol Life Sci. 2018. PMID: 29675785 Free PMC article. Review.
Cited by
-
Dendrobium nobile Lindl. Alkaloids Decreases the Level of Intracellular β-Amyloid by Improving Impaired Autolysosomal Proteolysis in APP/PS1 Mice.Front Pharmacol. 2018 Dec 18;9:1479. doi: 10.3389/fphar.2018.01479. eCollection 2018. Front Pharmacol. 2018. PMID: 30618767 Free PMC article.
-
Dendrobium nobile Lindl. alkaloids improve lipid metabolism by increasing LDL uptake through regulation of the LXRα/IDOL/LDLR pathway and inhibition of PCSK9 expression in HepG2 cells.Exp Ther Med. 2025 Jan 9;29(3):46. doi: 10.3892/etm.2025.12796. eCollection 2025 Mar. Exp Ther Med. 2025. PMID: 39885913 Free PMC article.
-
Dendrobium Alkaloids Promote Neural Function After Cerebral Ischemia-Reperfusion Injury Through Inhibiting Pyroptosis Induced Neuronal Death in both In Vivo and In Vitro Models.Neurochem Res. 2020 Feb;45(2):437-454. doi: 10.1007/s11064-019-02935-w. Epub 2019 Dec 21. Neurochem Res. 2020. PMID: 31865520
-
Recent progress in the role of autophagy in neurological diseases.Cell Stress. 2019 Apr 29;3(5):141-161. doi: 10.15698/cst2019.05.186. Cell Stress. 2019. PMID: 31225510 Free PMC article. Review.
-
The PI3K/Akt signaling axis in Alzheimer's disease: a valuable target to stimulate or suppress?Cell Stress Chaperones. 2021 Nov;26(6):871-887. doi: 10.1007/s12192-021-01231-3. Epub 2021 Aug 13. Cell Stress Chaperones. 2021. PMID: 34386944 Free PMC article. Review.
References
-
- Frozza RL, Horn AP, Hoppe JB, et al. A comparative study of β‐amyloid peptides Aβ1‐42 and Aβ25‐35 toxicity in organotypic hippocampus slice cultures. Neurochem Res. 2009;34:295–303. - PubMed
-
- Annweiler C, Brugg B, Peyrin J, Bartha R, Beauchet O. Combination of memantine and vitamin D prevents axon degeneration induced by amyloid‐beta and glutamate. Neurobiol Aging. 2014;35:331–335. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

