Mitochondrial Ca2+ Uniporter Is a Mitochondrial Luminal Redox Sensor that Augments MCU Channel Activity
- PMID: 28262504
- PMCID: PMC5357178
- DOI: 10.1016/j.molcel.2017.01.032
Mitochondrial Ca2+ Uniporter Is a Mitochondrial Luminal Redox Sensor that Augments MCU Channel Activity
Abstract
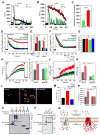
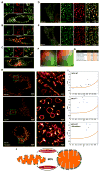
Ca2+ dynamics and oxidative signaling are fundamental mechanisms for mitochondrial bioenergetics and cell function. The MCU complex is the major pathway by which these signals are integrated in mitochondria. Whether and how these coactive elements interact with MCU have not been established. As an approach toward understanding the regulation of MCU channel by oxidative milieu, we adapted inflammatory and hypoxia models. We identified the conserved cysteine 97 (Cys-97) to be the only reactive thiol in human MCU that undergoes S-glutathionylation. Furthermore, biochemical, structural, and superresolution imaging analysis revealed that MCU oxidation promotes MCU higher order oligomer formation. Both oxidation and mutation of MCU Cys-97 exhibited persistent MCU channel activity with higher [Ca2+]m uptake rate, elevated mROS, and enhanced [Ca2+]m overload-induced cell death. In contrast, these effects were largely independent of MCU interaction with its regulators. These findings reveal a distinct functional role for Cys-97 in ROS sensing and regulation of MCU activity.
Keywords: EMRE; MCU; MCUR1; MICU1; MICU2; bioenergetics; calcium; gluathionylation; mitochondria; reactive oxygen species.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Molecular nature and physiological role of the mitochondrial calcium uniporter channel.Am J Physiol Cell Physiol. 2021 Apr 1;320(4):C465-C482. doi: 10.1152/ajpcell.00502.2020. Epub 2020 Dec 9. Am J Physiol Cell Physiol. 2021. PMID: 33296287 Free PMC article. Review.
-
MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics.Cell Rep. 2016 May 24;15(8):1673-85. doi: 10.1016/j.celrep.2016.04.050. Epub 2016 May 12. Cell Rep. 2016. PMID: 27184846 Free PMC article.
-
Coupled transmembrane mechanisms control MCU-mediated mitochondrial Ca2+ uptake.Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21731-21739. doi: 10.1073/pnas.2005976117. Epub 2020 Aug 14. Proc Natl Acad Sci U S A. 2020. PMID: 32801213 Free PMC article.
-
Analysis of the structure and function of EMRE in a yeast expression system.Biochim Biophys Acta. 2016 Jun;1857(6):831-9. doi: 10.1016/j.bbabio.2016.03.019. Epub 2016 Mar 18. Biochim Biophys Acta. 2016. PMID: 27001609
-
The mitochondrial Ca2+ uniporter: regulation by auxiliary subunits and signal transduction pathways.Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C67-80. doi: 10.1152/ajpcell.00319.2015. Epub 2016 Apr 27. Am J Physiol Cell Physiol. 2016. PMID: 27122161 Free PMC article. Review.
Cited by
-
Contribution of Mitochondrial Reactive Oxygen Species to Chronic Hypoxia-Induced Pulmonary Hypertension.Antioxidants (Basel). 2023 Nov 30;12(12):2060. doi: 10.3390/antiox12122060. Antioxidants (Basel). 2023. PMID: 38136180 Free PMC article.
-
Contribution of Oxidative Stress Induced by Sonodynamic Therapy to the Calcium Homeostasis Imbalance Enhances Macrophage Infiltration in Glioma Cells.Cancers (Basel). 2022 Apr 18;14(8):2036. doi: 10.3390/cancers14082036. Cancers (Basel). 2022. PMID: 35454942 Free PMC article.
-
The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology.Front Cell Dev Biol. 2018 Sep 4;6:96. doi: 10.3389/fcell.2018.00096. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30234109 Free PMC article. Review.
-
Cardiomyocyte Na+ and Ca2+ mishandling drives vicious cycle involving CaMKII, ROS, and ryanodine receptors.Basic Res Cardiol. 2021 Oct 14;116(1):58. doi: 10.1007/s00395-021-00900-9. Basic Res Cardiol. 2021. PMID: 34648073 Free PMC article.
-
Redox regulation of UPR signalling and mitochondrial ER contact sites.Cell Mol Life Sci. 2024 Jun 7;81(1):250. doi: 10.1007/s00018-024-05286-0. Cell Mol Life Sci. 2024. PMID: 38847861 Free PMC article. Review.
References
-
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

