Developmental Sex Differences in the Metabolism of Cardiolipin in Mouse Cerebral Cortex Mitochondria
- PMID: 28262723
- PMCID: PMC5338321
- DOI: 10.1038/srep43878
Developmental Sex Differences in the Metabolism of Cardiolipin in Mouse Cerebral Cortex Mitochondria
Abstract
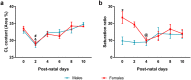
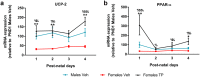
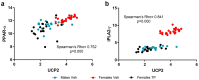
Cardiolipin (CL) is a mitochondrial-specific phospholipid. CL content and acyl chain composition are crucial for energy production. Given that estradiol induces CL synthesis in neurons, we aimed to assess CL metabolism in the cerebral cortex (CC) of male and female mice during early postnatal life, when sex steroids induce sex-dimorphic maturation of the brain. Despite the fact that total amount of CL was similar, its fatty acid composition differed between males and females at birth. In males, CL was more mature (lower saturation ratio) and the expression of the enzymes involved in synthetic and remodeling pathways was higher, compared to females. Importantly, the sex differences found in CL metabolism were due to the testosterone peak that male mice experience perinatally. These changes were associated with a higher expression of UCP-2 and its activators in the CC of males. Overall, our results suggest that the perinatal testosterone surge in male mice regulates CL biosynthesis and remodeling in the CC, inducing a sex-dimorphic fatty acid composition. In male's CC, CL is more susceptible to peroxidation, likely explaining the testosterone-dependent induction of neuroprotective molecules such as UCP-2. These differences may account for the sex-dependent mitochondrial susceptibility after perinatal hypoxia/ischemia.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Aoun M. et al.. Rat liver mitochondrial membrane characteristics and mitochondrial functions are more profoundly altered by dietary lipid quantity than by dietary lipid quality: effect of different nutritional lipid patterns. Br. J. Nutr. 107, 647–59 (2012). - PubMed
-
- Fry M. & Green D. E. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J. Biol. Chem. 256, 1874–80 (1981). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

