Increased circulating β2-adrenergic receptor autoantibodies are associated with smoking-related emphysema
- PMID: 28262783
- PMCID: PMC5338268
- DOI: 10.1038/srep43962
Increased circulating β2-adrenergic receptor autoantibodies are associated with smoking-related emphysema
Erratum in
-
Author Correction: Increased circulating β2-adrenergic receptor autoantibodies are associated with smoking-related emphysema.Sci Rep. 2020 Apr 10;10(1):6468. doi: 10.1038/s41598-020-63071-y. Sci Rep. 2020. PMID: 32277098 Free PMC article.
Abstract
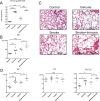
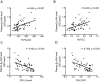
Smoking is a dominant risk factor for chronic obstructive pulmonary disease (COPD) and emphysema, but not every smoker develops emphysema. Immune responses in smokers vary. Some autoantibodies have been shown to contribute to the development of emphysema in smokers. β2-adrenergic receptors (β2-ARs) are important targets in COPD therapy. β2-adrenergic receptor autoantibodies (β2-AAbs), which may directly affect β2-ARs, were shown to be increased in rats with passive-smoking-induced emphysema in our current preliminary studies. Using cigarette-smoke exposure (CS-exposure) and active-immune (via injections of β2-AR second extracellular loop peptides) rat models, we found that CS-exposed rats showed higher serum β2-AAb levels than control rats before alveolar airspaces became enlarged. Active-immune rats showed increased serum β2-AAb levels, and exhibited alveolar airspace destruction. CS-exposed-active-immune treated rats showed more extensive alveolar airspace destruction than rats undergoing CS-exposure alone. In our current clinical studies, we showed that plasma β2-AAb levels were positively correlated with the RV/TLC (residual volume/total lung capacity) ratio (r = 0.455, p < 0.001) and RV%pred (residual volume/residual volume predicted percentage, r = 0.454, p < 0.001) in 50 smokers; smokers with higher plasma β2-AAb levels exhibited worse alveolar airspace destruction. We suggest that increased circulating β2-AAbs are associated with smoking-related emphysema.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
[Apoptosis of alveolar epithelial cells and pulmonary vascular endothelial cells in chronic obstructive pulmonary disease].Zhonghua Jie He He Hu Xi Za Zhi. 2008 Aug;31(8):581-5. Zhonghua Jie He He Hu Xi Za Zhi. 2008. PMID: 19080401 Chinese.
-
Expression of β1- and β2-adrenergic receptors in the lungs and changes in the levels of corresponding autoantibodies in an aged rat model of heart failure.Int J Mol Med. 2016 Dec;38(6):1933-1939. doi: 10.3892/ijmm.2016.2786. Epub 2016 Oct 24. Int J Mol Med. 2016. PMID: 27779651
-
Role of Cigarette Smoke-Induced Aggresome Formation in Chronic Obstructive Pulmonary Disease-Emphysema Pathogenesis.Am J Respir Cell Mol Biol. 2015 Aug;53(2):159-73. doi: 10.1165/rcmb.2014-0107OC. Am J Respir Cell Mol Biol. 2015. PMID: 25490051 Free PMC article.
-
[Quantitative analysis of emphysema and air trapping at inspiratory and expiratory phase multi-slice spiral CT scan in smokers: correlation with pulmonary function test].Zhonghua Yi Xue Za Zhi. 2018 May 22;98(19):1467-1473. doi: 10.3760/cma.j.issn.0376-2491.2018.19.003. Zhonghua Yi Xue Za Zhi. 2018. PMID: 29804412 Chinese.
-
Inflammatory cells and chronic obstructive pulmonary disease.Curr Drug Targets Inflamm Allergy. 2005 Dec;4(6):607-18. doi: 10.2174/156801005774912824. Curr Drug Targets Inflamm Allergy. 2005. PMID: 17305517 Review.
Cited by
-
Pre-existing self-reactive IgA antibodies associated with primary graft dysfunction after lung transplantation.Transpl Immunol. 2020 Apr;59:101271. doi: 10.1016/j.trim.2020.101271. Epub 2020 Jan 30. Transpl Immunol. 2020. PMID: 32007544 Free PMC article.
-
Aging, tobacco use and lung damages.Tunis Med. 2022 avril;100(4):295-302. Tunis Med. 2022. PMID: 36155900 Free PMC article.
-
Autoantibodies in Chronic Obstructive Pulmonary Disease.Front Immunol. 2018 Jan 25;9:66. doi: 10.3389/fimmu.2018.00066. eCollection 2018. Front Immunol. 2018. PMID: 29422903 Free PMC article. Review.
-
Role of melatonin as an SIRT1 enhancer in chronic obstructive pulmonary disease induced by cigarette smoke.J Cell Mol Med. 2020 Jan;24(1):1151-1156. doi: 10.1111/jcmm.14816. Epub 2019 Nov 25. J Cell Mol Med. 2020. PMID: 31762195 Free PMC article.
-
Set Up for Failure: Pre-Existing Autoantibodies in Lung Transplant.Front Immunol. 2021 Aug 11;12:711102. doi: 10.3389/fimmu.2021.711102. eCollection 2021. Front Immunol. 2021. PMID: 34456920 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

